抗菌肽(antibacterial peptides,AMPs)是具有抗菌活性的生物活性肽,一般由10~60个氨基酸残基组成,为双亲结构阳离子肽,具有热稳定性好、抗菌活性高、特异性强、哺乳动物细胞毒性小且不易产生耐药性等特性,一级结构中的氨基端常富含亲水性氨基酸,羧基端富含疏水性氨基酸,在细胞膜脂质疏水性环境中可形成α-螺旋和β-折叠等二级结构构象,对细菌、真菌和病毒,甚至癌细胞都有较好的抑制活性[1-2],在生物体内抑制致病微生物增殖、提升主动防御及增强免疫等生理活动发挥重要作用,有望成为抗菌药物的理想替代品[3]。抗菌肽根据所带电荷不同可分为阳离子抗菌肽和阴离子抗菌肽,根据来源不同可分为昆虫抗菌肽、哺乳动物抗菌肽、两栖动物抗菌肽、鱼类抗菌肽及微生物抗菌肽等,按结构不同可分为含α-螺旋结构抗菌肽、含β-折叠结构抗菌肽和其他结构抗菌肽等[4-5]。近些年,由于抗菌肽特殊的抑菌机理与活性及潜在抗菌药物替代性而备受关注并取得大量研究成果。本文简要介绍了抗菌肽分类,综述了抗菌肽一级结构与空间结构特点及抗菌肽结构改造策略,并对抗菌肽结构改造优化策略进行了展望,旨在为抗菌肽结构优化策略的选择提供理论参考,并推动抗菌肽的理论与应用研究。
1 抗菌肽的分子结构
1.1 一级结构
抗菌肽常为含有亲水性与疏水性区域的双亲结构,其一级结构的-NH2端富含赖氨酸(Lys)、精氨酸(Arg)和组氨酸(His)等亲水性氨基酸,而-COOH端常富含甘氨酸(Gly)、丙氨酸(Ala)和缬氨酸(Val)等疏水性氨基酸且被酰胺化[6],氨基酸残基数量一般少于100个,亲水与疏水氨基酸比例及氨基酸序列呈现多样化,形成完整的疏水面和亲水面,有利于抗菌肽与细胞膜(脂)蛋白结合,形成跨膜通道,改变细胞膜通透性而发挥抗菌活性[4,7]。所有植物抗菌肽都富含半胱氨酸(Cys)和-S-S-键[8],抗菌肽一级结构序列中Cys和Gly含量高于非抗菌肽,天冬氨酸(Asp)和谷氨酸(Glu)含量偏低[4]。抗菌肽一级结构常不稳定,在细胞膜脂质疏水环境中可折叠形成特定的稳定的空间结构或构象,结构疏水性对抑菌活性与溶血毒性有重要影响[9]。
1.2 二级结构
1.2.1 α-螺旋结构
α-螺旋是抗菌肽最常见且目前研究最广泛的二级结构构象,含α-螺旋抗菌肽在水溶液中大多为无序状态,当与三氟乙醇、高于临界胶束浓度的去污剂和表面活性剂(如十二烷基硫酸钠(sodium dodecyl sulfate,SDS)、胶束和脂质体)接触时会形成特定α-螺旋结构,两个相邻氨基酸残基距离约为0.15 nm,相对于中心的角度约为100°(俯视),常存在脯氨酸(Pro)或Gly形成的铰链区,无分子内二硫键,有广谱抗菌性,其氨基端和羧基端分别含亲水性和疏水性氨基酸残基,为双亲结构,可通过静电吸引结合到细胞膜脂质疏水区发挥抑菌活性[4],螺旋性有利于穿膜和破坏细胞膜结构,但过高螺旋性会增加细胞毒性[4,10]。α-螺旋抗菌肽结构[11]和作用机制[12]如下:
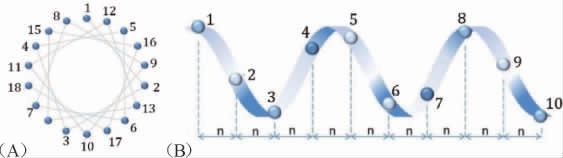
†:(A)抗菌肽螺旋轮投影(俯视),序列中相邻两个氨基酸角为100°,虚线表示一级结构中的相邻两个氨基酸;(B)抗菌肽的侧链结构图,两个相邻氨基酸的距离n为0.15 nm。
1.2.2 β-折叠结构
β-折叠为反平行结构且存在分子内二硫键,二硫键对维持抗菌肽β-折叠结构至为关键[4],能增强抗菌肽膜穿透性,含有由两个反平行折叠构成的具有较好保守性的γ-核心区域(gamma-core motif,γ-CM)[13];含β-折叠结构抗菌肽几乎都含有保守且形成二硫键的半胱氨酸残基,二硫键能增加抗菌肽的结构稳定性和酶耐受性,防御素是β-折叠结构抗菌肽典型代表[14]。
1.2.3 β-发夹结构与无规则卷曲结构
β-发夹结构抗菌肽-COOH末端含有1个分子内二硫键,-NH2端为线性结构,也为双亲结构,β-发夹结构可改变细胞膜通透性而导致细胞坏死[4,15]。无规则卷曲抗菌肽结构中富含Gly和Pro等氨基酸,通常不含Cys,常形成无序线性卷曲结构,在细胞质中发挥抗菌活性,可与胞内生物大分子结合,对脱氧核糖核酸(deoxyribonucleic acid,DNA)复制、核糖核酸(ribonucleic acid,RNA)转录与基因调控等关键过程发挥重要影响,干扰胞内蛋白质空间结构形成及酶催化活性发挥[6]。
1.3 三级与四级结构
抗菌肽疏水区域在特定疏水环境中可形成稳定的三级或四级空间结构[9]。如抗菌肽Gaegurin 4处于亲水环境时不表现特定二级结构,但在脂质疏水环境时却表现出了α-螺旋与β-折叠空间结构,核磁共振(nuclear magnetic resonance,NMR)研究发现,在亲水性环境时没有形成稳定的三级空间结构,但在三氟乙醇水溶液中和体积分数为80%甲醇水溶液等疏水环境中表现出不同的三维空间结构[16]。
2 抗菌肽结构优化与改造策略
2.1 改变净正电荷数量与分布
阳离子抗菌肽主要是因为富含Arg、Lys和His等碱性氨基酸残基,正电荷多寡能影响抗菌肽与细胞膜带负电荷磷脂的静电吸附力及抗菌肽抑菌浓度[17]。用精氨酸(Arg)和Lys替换Tenecin1结构中α-螺旋区域的氨基酸残基能增加净电荷并显著增强抗菌活性[18];用Lys替换抗菌肽Rr-AFP2第39位谷氨酰胺(Gln)可增强抗菌活性,而用Gln替换44位Lys后抗菌活性降低[4];JIANG Z Q等[19]将LV13K改造成净电荷-5至+10的系列抗菌肽并发现,净电荷-5至+8的系列抗菌肽抑菌活性随净电荷增加而增加,净电荷为+8时抑菌活性最大,净电荷增加至+10时,抑菌活性下降且溶血活性明显增强。抗菌活性与净电荷并非简单正相关关系主要是因为净电荷过高时,抗菌肽分子间静电荷斥力超过抗菌肽与细胞膜磷脂的静电吸附力,抗菌活性降低且增加细胞溶血活性。电荷和抗菌活性出现间接或完全反向关系可能是由于抗菌肽与磷脂头基团之间极强的相互作用阻止该肽易位到内膜中[4]。研究发现,含His残基抗菌肽表现pH依赖性杀菌活性,在His残基被质子化的酸性pH下观察到了抗菌活性显著提高,主要是因为增加抗菌肽的净电荷能增强与细胞膜表面相互作用,酸性pH值产生类似金属离子的作用[20]。ZHANG S K等[21]通过置换正电荷残基以改变净电荷多寡及正电荷残基分布改造抗菌肽AR-23,研究发现净电荷增加能影响抗菌活性,但与正电荷残基数量无关。因此,适当增加抗菌肽净电荷数量和优化正电荷分布可提高抗菌肽的抗菌活性。
2.2 调整抗菌肽的α-螺旋度
抗菌肽氨基端形成的α-螺旋空间构象是双亲结构形成的重要先决条件,对抗菌活性有重要影响,用正电荷氨基酸残基取代非极性面能消除α-螺旋度并降低溶血活性,但对抗菌活性影响较小[22]。抗菌肽Melittin氨基端Gly残基替换为Leu或去除能提高α-螺旋度,抗菌活性和溶血活性均有所增加[23]。GAGNON M C等[17]研究发现,在净正电荷和肽链长度不变的前提下,抑菌活性随α-螺旋度消失而降低;用D-氨基酸替换L-氨基酸能降低抗菌肽α-螺旋度并降低溶血活性,是调整抗菌肽α-螺旋度的重要策略[24]。OREN Z等[25]用D-型氨基酸替代抗菌肽melittin B的4个氨基酸残基发现α-螺旋度降低了79.45%,抑菌活性不变,而动物细胞毒性却明显降低;PAPO N等[26]研究发现,D-型氨基酸比L-氨基酸更能有效降低动物细胞毒性和增强抑菌活性。但需注意,α-螺旋肽虽有利穿膜且抗菌活性较高,但螺旋性过高会增强细胞毒性[27],因此,需权衡α-螺旋度与抗菌活性及细胞毒性的关系。
2.3 提高抗菌肽蛋白酶耐受性
蛋白酶耐受性是抗菌肽在生物体内应用的重要结构基础。将抗菌肽结构中带正电荷的Arg用其他带电荷的非天然氨基酸(如L-鸟氨酸和L-高精氨酸)或D-氨基酸取代[28],或通过N末端乙酰化阻止氨肽酶活性可增强抗菌肽蛋白酶稳定性[29]。ZHAO Y Y等[30]将富含Lys的蜂毒肽抗菌肽(MPI)中所有氨基酸均用D-氨基酸取代,研究发现,改造后的D-MPI与MPI抗菌活性差异不显著,且具备较好的胰蛋白酶耐受性,但制备成本极高;抗菌肽环化也能增强抗菌肽蛋白酶耐受性,环状结构和分子内二硫键常赋予环化抗菌肽耐热、耐酶解且抑菌活性高等理化性质[31],如人防御素二硫键环化可极大提高其血清稳定性,环化过程应注意保留α-螺旋空间结构,应选择在i和i+4环化(第1和第4个氨基酸被环化),避免i和i+6环化[32];拟肽是通过修饰肽链主侧链骨架(如改变主链通过氮而非α-碳原子键合)增强消化耐受性而保留侧链空间结构获得的类似肽聚合物[33],拟肽环化还能增强细胞膜渗透性和抑菌活性[34]。
2.4 改变疏水性及平均疏水矩大小
疏水性强弱是决定抗菌肽进入细胞膜磷脂层和膜通透性重要的结构基础,抗菌肽疏水性太低,与细胞膜脂质亲和力较弱,膜穿透性较差,相反则易造成抗菌肽自我聚集和增强细胞毒性及溶血活性[35]。疏水性在一定范围内与抑菌活性呈良好正相关关系,引入疏水基团能拓展抗菌谱和增强抗菌活性,但疏水性过强易降低细胞选择性和增强哺乳动物细胞毒性,不宜超过50%[36]。CHEN Y X等[37]研究确定了抗菌肽V13KL对人类红细胞有良好抗菌活性和较低溶血活性的最佳疏水性,通过改变序列增加或降低疏水值后抗菌活性显著降低,可能是因为具有较高疏水性的抗菌肽更容易深入到红细胞膜疏水中心[37]。平均疏水矩对抗菌活性的影响要大于螺旋度和疏水性,合理调节平均疏水矩有利于提高抑菌活性[38]。LEE K H等[39]将抗菌肽HP第16位Gln和第18位Asp用Trp替换,疏水性与抗菌活性均提高且呈良好正相关,平均疏水矩降低后,抗菌活性与溶血能力随之降低[40]。抗菌肽结构优化时应注意疏水性和疏水矩合适取值区间,要权衡抗菌活性、抗菌谱与哺乳动物细胞毒性的关系。
2.5 改变抗菌肽的两亲性
两亲性是指抗菌肽结构内亲水和疏水残基或结构域的相对丰度,可认为是在一级序列及空间结构上阳离子残基和疏水残基之间的平衡。普遍观点认为,增加α-螺旋抗菌肽的两亲性可增强抗菌活性和降低红细胞毒性[41],但也有研究认为,两亲性增加会降低对红细胞的细胞毒性[42];β-折叠抗菌肽反向平行的β-折叠链也具有两亲性,通过二硫键形成稳定构象,当从水溶液进入细胞膜时不发生主要结构改变,增强β-折叠抗菌肽的两亲性导致其溶血毒性增加,但对抗菌活性影响较小,表明两亲性和溶血之间存在相关性[43],而电荷和两亲性增加的indolicidin 类似物在抗菌活性不变的条件下却显示低溶血毒性[44],因此,抗菌肽两亲性与抗菌活性及红细胞毒性关系复杂,尚不明确。
2.6 改变氨基酸残基位置及肽链长度
超过50%抗菌肽首位氨基酸残基为Gly,富含Arg和Val的抗菌肽由于和细胞膜脂质层有较强的静电吸附力而发挥良好抗菌活性,富含Arg有助于膜穿孔,富含Val有助于形成β-折叠空间构象和抗菌活性发挥,富含Pro和Gly对稳定结构与抗菌活性发挥有重要作用[45-46]。LEE D G等[47]逐一剔除抗菌肽P1的C端和N端氨基酸,结果发现Pro是影响抗菌活性的关键氨基酸残基;MISHRA A K等[48]研究富含Try的抗菌肽发现,Try残基位置改变可影响抗菌活性,而改变Try残基数对抗菌活性基本无影响;用Lys取代粘菌素序列(GIHDILKYGKPS)的His残基会导致其抗菌活性降低,在N端添加Try残基并同时用带正电荷的Arg置换某些残基可提高其杀菌活性[49]。合适肽链长度也是维持抗菌肽抑菌活性重要因素[7,50],含较多正电荷的较长抗菌肽抑菌效果较好,而短链抗菌肽的正电荷增加其抑菌活性反而降低[51]。
2.7 构建杂合抗菌肽
WANG L N等[52]应用基因工程手段将attacin和thanatin融合构建杂合肽attacin-thanatin并在大肠杆菌中成功表达,该杂合肽抗菌活性比单一attacin要高,且对猪红细胞无任何毒性,对大肠杆菌和金黄色葡萄球菌等也有较好抑菌活性;张宏刚等[53]构建了杂合抗菌肽Mel-MytB并在毕氏酵母中成功表达,该杂合肽具有广谱抗菌活性,对细菌有较强抑菌活性且耐热和耐酸;KIM H K等[54]制备的杂合抗菌肽HP-ma抑菌活性是HP和magainin的2~32倍,比magainin具有更好的膜裂解活性。因此,通过构建杂合抗菌肽是提高抗菌活性和拓宽抗菌谱的重要策略[55]。
2.8 降低哺乳动物细胞毒性
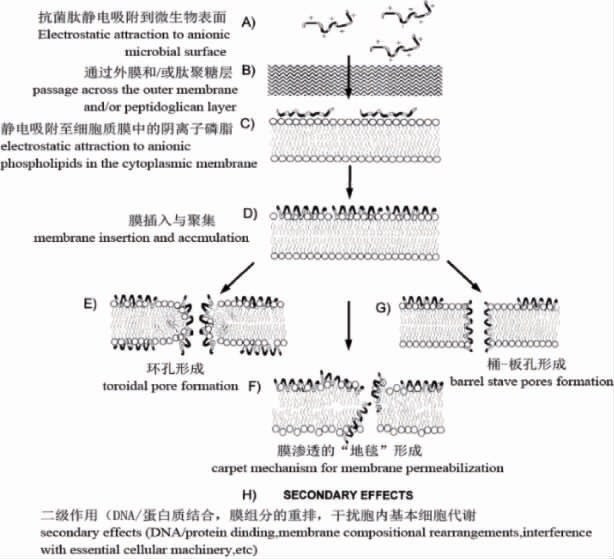
抗菌肽常具有一定细胞选择性,特别是阳离子抗菌肽,主要是因为细菌细胞膜表面含有比哺乳动物细胞膜更多的负电荷,对阳离子抗菌肽更敏感,G-细菌外膜脂多糖(lipopolysaccharide,LPS)和G+细菌肽聚糖磷壁酸等阴离子分子也可通过静电吸附与阳离子抗菌肽结合[56]。酸性磷脂通常位于哺乳动物细胞质膜内部单层中,外部单层主要由两性离子磷脂酰胆碱和鞘磷脂构成,带负电荷的神经节苷脂以次要成分存在,抗菌肽疏水表面与细胞表面上两性离子磷脂间疏水作用起主要作用,哺乳动物细胞中稳定膜的胆固醇可保护免受抗菌肽攻击[57]。抗菌肽细胞选择性分子机理[58]如下:
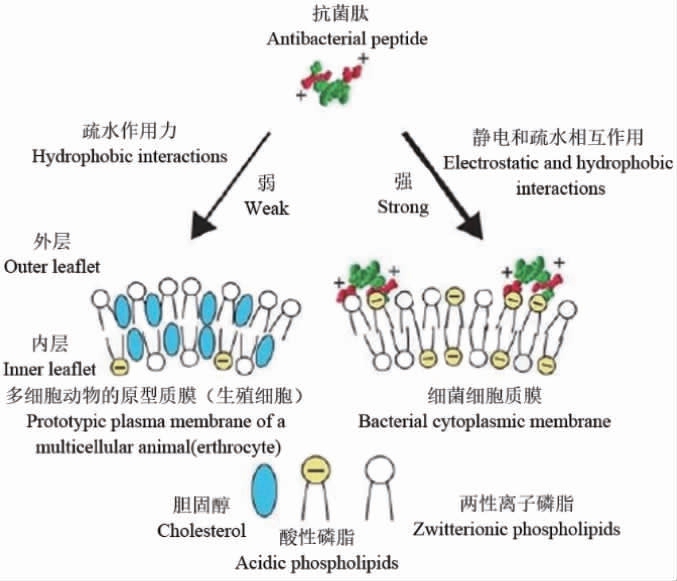
LEE H等[56]基于抗菌肽tritrpticin和indolicidin序列对细胞选择性差且具有很强溶血活性的富含Trp氨基酸残基抗菌肽进行结构改造,构建了具有对称氨基酸序列的α-抗菌肽,研究发现,具有对称氨基酸序列的α-抗菌肽(α-TRAMPs)对G-和G+细菌有很强抑菌活性且对哺乳动物细胞表现极低细胞毒性和溶血活性。天然抗菌肽通过结构优化可降低哺乳动物细胞毒性,如cathelicidin铰链区Pro用Gly取代溶血性降低了61.54%,Leu取代后无溶血活性,结构优化也可降低哺乳动物细胞毒性,特别是肾毒性[59];添加辅助基团也可以增强抗菌活性并减弱溶血活性,如在富含Try和Arg的人工合成抗菌肽氨基端接技有机金属FcCO或RcCO能增强抗菌活性并降低溶血性[60],聚乙二醇修饰也能增强抗菌肽组织相溶性和肺组织毒性且抗菌活性不变[61]。
2.9 制备金属抗菌肽提升抗菌活性与拓展抗菌谱
多肽是金属离子配位螯合通用配体,多肽主链肽键中-NH2端、-COOH端和N原子及侧链相关基团可与金属离子配位螯合并增强抗菌活性和结构稳定性[62-63]。如具有ATCUN基序的富组蛋白与Fe2+结合导致α-螺旋结构丧失,抗真菌活性大大降低,而与Cu2+或Ni2+配位可增强抗真菌活性,与Zn2+配位螯合可增强抗菌活性[64]。铁调素为β-折叠肽,人铁调素25与Cu2+螯合能增强对金黄色葡萄球菌和铜绿假单胞菌的抑菌活性,因为Cu2+结合可诱导肽构象变化,增加肽对细胞内靶标的抗菌活性,螯合Fe2+也能增强抗菌活性[65]。与阳离子抗菌肽作用机理不同,阴离子抗菌肽也可利用金属配位离子与细胞膜结构中带负电荷组分形成阳离子盐桥而穿透膜,若抗菌肽易位于细胞质中,还可附着在核糖体或抑制核糖核酸酶活性,如Theromyzin[66]。
3 展望
随着结构生物学与生物信息学技术的快速发展,高抑菌活性的抗菌肽构-效关系结构精准研究将成为可能。抗菌肽结构优化可围绕以下几个关键问题展开:(1)抗菌肽结构优化设计与精准修饰。天然抗菌肽常存在构-效关系非最优情况,如阳离子抗菌肽虽然对哺乳动物细胞毒副作用少且有一定细胞选择性[36],但结构改造时需注意抗菌肽疏水性和疏水矩参数的优化,疏水性太强易造成自我聚集和膜渗透能力减弱,且溶血活性和细胞毒性增强,一般抗菌肽净正电荷保持在+4~+8较为合适,并保持合适肽链长度,可采取氨基酸残基替换法改变肽疏水性并控制合适净正电荷和链长[21];α-螺旋肽动物细胞毒性小且抑菌活性高,可用D-型氨基酸替代L-氨基酸构建合适的α-螺旋度以降低细胞毒性和提高抑菌活性[6,67];(2)获得抗菌肽构-效关系精准生物学信息。开展组学技术与抗菌活性构-效关系研究,定位抗菌活性关键氨基残基位点及空间结构与抗菌活性的关联,构建抗菌肽构-效关系数据库,基于大数据分析抗菌肽与细胞膜、胞内核酸与蛋白质(酶)以及线粒体等作用机制,或用仿生模拟技术研究抗菌肽在非均相生物体系中与受体分子的作用机理、模拟生物体中胃肠耐受性及体内转运动力学,以及模拟抗菌肽在生物体内的作用机制,获得抗菌肽精准的构-效生物学信息[68];(3)开展抗菌肽耐药机制的创新研究。最新研究发现[69],在Phop/Q革兰氏阴性菌等细菌中发现可通过抗菌肽二元调控系统,或修饰细胞壁(膜)带负电荷组分,或分泌诱捕阳离子抗菌肽物质阻止抗菌肽吸附至细胞壁膜,或分泌可降解抗菌肽的蛋白酶,或通过外排泵将抗菌肽排出胞外等耐药机制[70]。近年来,抗菌肽研究主要以细菌细胞膜作为效应靶点[71],但长期使用抗菌肽也可能存在耐药性[72],因此,除将细胞膜作为抗菌肽筛选效应靶点外,也要考虑基于细胞壁、线粒体、胞内生物大分子合成抑制、物质与能量代谢关键酶靶点等开展抗菌肽抑菌机制的系统研究[4];(4)改善抗菌肽生物体内的输送与转运效能。如将抗菌肽以共价(或非共价)结合方式与输送载体(如钠米金、石墨烯和量子点等)结合,或用脂质(或表面活性剂)包埋,或用聚合材料对抗菌肽进行负载[73]增强抗菌活性与酶耐受性且降低溶血毒性与细胞毒性[74-75],同时,开展纳米缓释材料等抗菌肽包埋材料的研究,通过包封抗菌肽并使抗菌肽缓慢释放可维持一定的血药浓度,从而提升抗菌效果。
[1]WU M H,CHEN Q,WANG Y D,et al.Structural modification and antitumor activity of antimicrobial peptide HYL[J].Chin Chem Lett,2020,31(5):1288-1292.
[2]DATTA S,ROY A.Antimicrobial peptides as potential therapeutic agents:a review[J].Int J Pept Res Ther,2021,27:555-577.
[3]WANG J J,DOU X J,SONG J,et al.Antimicrobial peptides:Promising alternatives in the post feeding antibiotic era[J].Med Res Rev,2019,39(3):831-859.
[4]LI S Q,WANG Y J,XUE Z H,et al.The structure-mechanism relationship and mode of actions of antimicrobial peptides:A review[J].Trends Food Sci Tech,2021,109:103-115.
[5]SILVA S,VALE N.Cationic antimicrobial peptides for tuberculosis:A mini-review[J].Curr Protein Pept Sc,2019,20(9):885-892.
[6]AHMED T A E,HAMMAMI R.Recent insights into structure-function relationships of antimicrobial peptides[J].J Food Biochem,2018,43(1):e12546.
[7] PANDIT G,CHOWDHURY N,MOHID S A,et al.Effect of secondary structure and side chain length of hydrophobic amino acid residues on the antimicrobial activity and toxicity of 14-residue-long de novo AMPs[J].Chem Med Chem,2021,16(2):355-367.
[8]TAM J T,WANG S J,WONG K H,et al.Antimicrobial peptides from plants[J].Pharmaceuticals,2015,8(4):711-757.
[9]GONG H N,ZHANG J,HU X Z,etal.Hydrophobic control of the bioactivity and cytotoxicity of de Novo designed antimicrobial peptides[J].ACS Appl Mater Interfaces,2019,11(38):34609-34620.
[10]PATHAK N,SALAS-AUVERT R,RUCHE G,etal.Comparison of the effects of hydrophobicity,amphiphilicity,and alpha-helicity on the activities of antimicrobial peptides[J].Proteins,2020,22(2):182-186.
[11]BAHAR A,REN D.Antimicrobial peptides[J].Pharmaceuticals,2013,6(12):1543-1575.
[12]TOSSI A,SANDRI L,GIANGASPERO A.Amphipathic,α-helical antimicrobial peptides[J].Peptide Sci,2015,55(21):4-30.
[13]SONDEREGGER C,VÁRADI G,GALGÓCZY L,et al.The evolutionary conserved γ-core motifinfluencesthe anti-Candida activityofthe Penicillium chrysogenum antifungal protein PAF[J].Front Microbiol,2018,9:1655.
[14]PARDUCHO K R,BEADELL B,YBARRA T K,et al.The antimicrobial peptide human beta-defensin 2 inhibits biofilm production of Pseudomonas aeruginosa without compromising metabolic activity[J].Front Immunol,2020,11:805.
[15] DONG N,WANG C S,ZHANG T T,et al.Bioactivity and bactericidal mechanism of histidine-rich β-hairpin peptide against gram-negative bacteria[J].Int J Mol Sci,2019,20(16):3954.
[16]KIM H J,KIM S S,LEE M H,et al.Role of C-terminal heptapeptide in poreforming activity of antimicrobial agent,gaegurin 4[J].J Pept Res,2010,64(4):151-158.
[17] GAGNON M C,STRANDBERG E,GRAU-CAMPISTANY A,et al.Influence of the length and charge on the activity of alpha-helical amphipathic antimicrobial peptides[J].Biochemistry,2017,56(11):1680-1695.
[18]AHN H S,CHO W,KANG S H,et al.Design and synthesis of novel antimicrobial peptides on the basis of α helical domain of Tenecin 1,an insect defensin protein,and structure-activity relationship study[J].Peptides,2006,27(4):640-648.
[19] JIANG Z Q,VASIL A I,HALE J D,et al.Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides[J].Biopolymers,2009,90(3):369-383.
[20]KACPRZYK L,RYDENGRD V,MÖRGELIN M,et al.Antimicrobial activity of histidine-rich peptides is dependent on acidic conditions[J].Biochim Biophys Acta,2007,1768(11):2667-2680.
[21]ZHANG S K,SONG J W,GONG F,et al.Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity[J].Sci Rep,2016,6:27394.
[22] MITRA J B,SHARMA V K,KUMAR M,etal.Antimicrobial peptides:Vestiges of past or modern therapeutics?[J].Mini Rev Med Chem,2020,20(3):183-195.
[23]BLONDELLE S E,HOUGHTEN R A.Hemolytic and antimicrobial activities of the twenty-four individual omission analogs of melittin[J].Biochemistry,1991,30(19):4671-4678.
[24]CROSS L J,ENNIS M,KRAUSE E,et al.Influence of alpha-helicity,amphipathicity and D-amino acid incorporation on the peptide-induced mast cell activation[J].Eur J Pharmacol,1995,291(3):291-300.
[25] OREN Z,SHAI Y.Selective lysis of bacteria but not mammalian cells by diastereomers of melittin:structure-function study[J].Biochemistry,1997,36(7):1826-1835.
[26]PAPO N,OREN Z,PAG U.The consequence of sequence alteration of an amphipathic alpha-helical antimicrobial peptide and its diastereomers[J].J Biol Chem,2002,277(37):33913-33921.
[27] BOWIE J H,SEPAROVIC F,TYLER M.Host-defense peptides of Australian anurans.part 2.structure,activity,mechanism of action,and evolutionary significance[J].Peptides,2012,37(1):174-188.
[28]DOMHAN C,UHL P,KLEIST C,et al.Replacement of l-amino acids by d-amino acids in the antimicrobial peptide ranalexin and its consequences for antimicrobial activity and biodistribution[J].Molecules,2019,24(16):e2987.
[29]PAPANASTASIOU E A,HUA Q Y,SANDOUK A,et al.Role of acetylation and charge in antimicrobial peptides based on human β-defensin-3[J].Apmis,2010,117(7):492-499.
[30]ZHAO Y Y,ZHANG M,QIU S,et al.Antimicrobial activity and stability of the D-amino acid substituted derivatives of antimicrobial peptide polybia-MPI[J].AMB Express,2016,6:122.
[31] BERGEN G V D,STROET M,CARON B,et al.Curved or linear? Predicting the 3-dimensional structure of α-helical antimicrobial peptides in an amphipathic environment[J].FEBB Lett,2020,594(6):1062-1080.
[32]SHARMA M,SINGHVI I,ALI ZAINAB M,etal.Synthesis and biological evaluation of natural cyclic peptide[J].Future J Pharm Sci,2018,4(2):220-228.
[33]GREENRM,BICKER KL.Evaluation ofpeptoid mimics of short,lipophilic peptide antimicrobials[J].Int J Antimicrob Ag,2020,56(2):106048.
[34]ANDREEV K,MARTYNOWYCZ M W,IVANKIN A,et al.Cyclization improves membrane permeation by antimicro-bial peptoids[J].Langmuir,2016,32(48):12905-12913.
[35]HOLLMANN A,MARTÍNEZ M,NOGUERA M E,et al.Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptidemembrane interactions of three related antimicrobial peptides[J].Colloids Surf B Biointerfaces,2016,141:528-536.
[36]JIANG Z Q,KULLBERG B J,LEE H V D,et al.Effects of hydrophobicity on the antifungal activity of alpha-helical antimicrobial peptides[J].Chem Biol Drug Des,2008,72(6):483-495.
[37]CHEN Y X,GUARNIERI M T,VASIL A I,et al.Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides[J].Antimicrob Agents Chemother,2007,51(4):1398-1406.
[38] CHERRY M,HIGGINS S K,MELROY H,et al.Peptideswith the same composition,hydrophobicity,and hydrophobic moment bind to phospholipid bilayers with different affinities[J].J Phys Chem B,2014,118(43):12462-12470.
[39] LEE K H,LEE D G,PARK Y,et al.Interactions between the plasma membrane and the antimicrobial peptide HP(2-20)and its analogues derived from Helicobacter pylori[J].Biochem J,2006,394(Pt1):105-114.
[40] PATHAK N,SALAS-AUVERT R,RUCHE G,et al.Comparison of the effects of hydrophobicity,amphiphilicity,and alpha-helicity on the activities of antimicrobial peptides[J].Proteins,1995,22(2):182-186.
[41]CHEN Y X,MANT C T,FARMER S W,et al.Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index[J].J Biol Chem,2015,280(13):12316-12329.
[42]ZHANG S K,SONG J W,GONG F,et al.Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity[J].Sci Rep,2016,6:27394.
[43] EDWARDS I A,ELLIOTT A G,KAVANAGH A M,et al.Contribution of amphipathicity and hydrophobicity to the antimicrobial activity and cytotoxicity of β-hairpin peptides[J].ACS Infect Dis,2016,2(6):442-450.
[44]FALLA T J,HANCOCK R E.Improved activity of a synthetic indolicidin analog[J].Antimicrob Agents Chemother,1997,41(4):771-775.
[45] EDWARDS-GAYLE C J C,BARRETT G,ROY S,et al.Selective antibacterial activity and lipid membrane interactions of arginine-rich amphiphilic peptides[J].ACS Appl Bio Mater,2020,3(2):1165-1175.
[46]LI W Y,TAILHADES J,O'BRIEN-SIMPSON N M,et al.Proline-rich antimicrobial peptides:Potential therapeutics against antibiotic-resistant bacteria[J].Amino Acids,2014,46(10):2287-2294.
[47] LEE D G,HAHM K S,SHIN S Y.Structure and fungicidal activity of a synthetic antimicrobial peptide,P18,and its truncated peptides[J].Biotechnol Lett,2004,26(4):337-341.
[48]MISHRA A K,CHOI J,MOON E,et al.Tryptophan-rich and proline-rich antimicrobial peptides[J].Molecules,2018,23(4):815.
[49]CANTISANI M,FINAMORE E,MIGNOGNA E,et al.Structural insights into and activity analysis of the antimicro-bial peptide myxinidin[J].Antimicrob Agents Chemother,2014,58(9):5280-5290.
[50]PHAMBU N,ALMARWANI B,GARCIA A M,et al.Chain length effect on the structure and stability of antimicrobial peptides of the(RW)n series[J].Biophys Chem,2017,227:8-13.
[51] GAGNON M C,STRANDBERG E,GRAU-CAMPISTANY A,et al.Influence of the length and charge on the activity of α-helical amphipathic antimicrobial peptides[J].Biochemistry,2017,56(11):1680-1695.
[52]WANG L N,YU B,HAN G Q,et al.Design,expression and characterization of recombinant hybrid peptide Attacin-Thanatin in Escherichia coli[J].Mol Biol Rep,2010,37(7):3495-3501.
[53]张宏刚,吴剑良,李莉.杂合抗菌肽Mel-MytB 在毕赤酵母中的表达及其抗菌活性测定[J].中国畜牧兽医,2018,45(11):276-282.
[54] KIM H K,LEE D G,PARK Y,et al.Antibacterial activities of peptides designed as hybrids of antimicrobial peptides[J].Biotechnol Lett,2002,24:347-353.
[55]KLUBTHAWEE N,ADISAKWATTANA P,HANPITHAKPONG W,et al.A novel,rationally designed,hybrid antimicrobial peptide,inspired by cathelicidin and aurein,exhibits membrane-active mechanisms against Pseudomonas aeruginosa[J].Sci Rep,2020,10:e9117.
[56]LEE H,YANG S,SHIN S Y.Improved cell selectivity of symmetric α-helical peptides derived from trp-rich antimicrobial peptides[J].B Korean Chem Soc,2020,41(9):930-936.
[57]ZASLOFF M.Antimicrobial peptides of multicellular organisms[J].Nature,2002,415:389-395.
[58]NAGARAJAN D,ROY N,KULKARNI O,et al.Ω76:A designed antimicrobial peptide to combat carbapenem-and tigecycline-resistant Acinetobacter baumannii[J].Sci Adv,2019,5(7):1946.
[59]ALBADA H B,CHIRIAC A L,WENZEL M,et al.Modulating the activity of short arginine-tryptophan containing antibacterial peptides with N-terminal metallocenoyl groups[J].Beilstein J Org Chem,2012,8:1753-1764.
[60]MORRIS C J,BECK K,FOX M A,et al.Pegylation of antimicrobial peptides maintains the active peptide conformation,model membrane interactions,and antimicrobial activitywhile improving lung tissue biocompatibility following airway delivery[J].Antimicrob Agents Chemother,2012,56(6):3298-3308.
[61] LACHOWICZ J L,TORRE G D,CAPPAI R,et al.Metal self-assembly mimosine peptides with enhanced antimicrobial activity:towards a new generation of multitasking chelating agents[J].Dalton Trans,2020,49(9):2862-2879.
[62] DASHPER S G,O'BRIEN-SIMPSON N M,CROSS K,et al.Divalent metal cations increase the activity of the antimicrobial peptide kappacin[J].Antimicrob Agents Ch,2005,49(6):2322-2328.
[63] PURI S,LI R,RUSZAJ D,et al.Iron binding modulates candidacidal properties of salivary histatin 5[J].J Dent Res,2015,94(1):201-208.
[64]MAISETTA G,PETRUZZELLI R,BRANCATISANO F L,et al.Antimicrobial activity of human hepcidin 20 and 25 against clinically relevant bacterial strains:effect of copper and acidic pH[J].Peptides,2010,31(11):1995-2002.
[65]PAULMANN M,ARNOLD T,LINKE D,et al.Structure-activity analysis of the dermcidin-derived peptide DCD-1L,an anionic antimicrobial peptide present in human sweat[J].J Biol Chem,2012,287(11):8434-8443.
[66]DOMINGUES T M,PEREZ K R,RISKE K A.Revealing the mode of action of halictine antimicrobial peptides:A comprehensive study with model membranes[J].Langmuir,2020,36(19):5145-5155.
[67]TAN P,LAI Z H,ZHU Y J,et al.Multiple strategy optimization of specifically targeted antimicrobial peptide based on structure-activity relationships to enhance bactericidal efficiency[J].ACS Biomater Sci Eng,2020,6(1):398-414.
[68]OTTO M.Bacterial sensing of antimicrobial peptide[J].Contrib Microbiol,2019,16:136-149.
[69]陈武,定军,丁彦,等.病原微生物对抗菌肽抗性机制的研究进展[J].中国生物工程杂志,2012,32(5):97-106.
[70]EPAND R M,WALKER C,EPAND R F,et al.Molecular mechanisms ofmembrane targeting antibiotics[J].Biochim Biophys Acta,2016,1858(5):980-987.
[71]ANDERSSON D I,HUGHES D,KUBICEK-SUTHERLAND J Z.Mechanisms and consequences of bacterial resistance to antimicrobial peptides[J].Drug Resist Update,2016,26:43-57.
[72]COSTA J R,SILVA N C,SARMENTO B,et al.Delivery systems for antimicrobial peptides and proteins:towards optimization of bioavailability and targeting[J].Curr Pharm Biotechnol,2017,18(2):108-120.
[73] KUMAR P,SHENOI R,LAI B,et al.Conjugation of aurein 2.2 to HPG yields an antimicrobial with better properties[J].Biomacromolecules,2015,16(3):913-923.
[74] SCORCIAPINO M A,SERRA I,MANZO G,et al.Antimicrobial dendrimeric peptides:Structure,activity and new therapeutic applications[J].Int J Mol Sci,2017,18(3):542.