胞外多糖(exopolysaccharides,EPS)是由微生物在生长过程中产生并分泌到细胞外的多糖类物质[1]。目前,EPS已经在食品与医药行业广泛开发利用,如在食品行业中用于面包、乳制品等产品的生产加工[2];在医药方面应用于用于生物支架,药物开发等方向[3-4]。在已报道的EPS中,细菌纤维素(bacterial cellulose,BC)作为种植牙材料、伤口敷料和临时皮肤替代品,被大量研究,直接导致了其生物医学产品的商业化[2]。另一种EPS—黄原胶市场价值不断增长,预计2018~2027年全球黄原胶市场价值将增长1.5倍[5]。能产生EPS的微生物很多,其中乳酸菌(lactic acid bacteria,LAB)与人们的生产生活有紧密的联系,因此由乳酸菌代谢生产的EPS备受关注。
乳酸菌EPS是乳酸菌在生长代谢过程中分泌到细胞壁外的粘液多糖或荚膜多糖,其具有高黏度和优良的流变学特性,可作为天然安全的增稠剂、乳化剂和稳定剂用于改善食品的质地及口感,在一定程度上降低了传统增稠剂和稳定剂的使用,因而在食品和医药等工业生产中具有广泛的应用前景[6]。此外,作为公认安全的食品微生物,乳酸菌代谢产生的EPS对人体无毒副作用、来源安全可靠,且对人体健康有着重要的促进作用,如增强人体免疫力、抗肿瘤、抗炎和降血糖等[7]。能产生EPS的乳酸菌种类很多,这使得乳酸菌EPS的种类也很多、来源较广。同时,乳酸菌EPS的结构与活性也极具多样性。
因此,研究乳酸菌EPS的产生及其结构性质具有很强的应用潜力。但乳酸菌合成EPS受多种条件的影响,如培养条件、物理环境、菌株种类等,不同的乳酸菌在不同条件下产生的EPS的结构、产量、性质各不相同。因此,为了综合全面地了解乳酸菌EPS,本文从其产生菌、类型、代谢途径、活性、合成影响因素和应用等方面进行了归纳,旨在为EPS的研究应用提供较为全面的信息。
1 乳酸菌与EPS
1.1 产EPS的乳酸菌
目前,已有研究报道能产生的EPS的细菌有醋杆菌属(Acetobacter)、农杆菌属(Agrobacterium)、芽孢杆菌属(Bacillus)、布伦纳菌属(Brenneria)、地杆菌属(Geobacillus)、葡萄醋杆菌属(Gluconacetobacter)、盐单胞菌属(Halomonas)、乳酸菌属(Lactobacillus)、根瘤菌属(Rhizobium)、酵母菌属(Saccharomyces)、肌球菌属(Sarcina)、链球菌属(Strep tococcus)、黄单胞菌属(Xanthomonas)和发酵单胞菌属(Zymomonas)[8]。其中,乳酸菌是目前产EPS应用最为常见的细菌[9]。产EPS的乳酸菌主要有乳酸菌属(Lactobacillus)、明串珠菌属(Leuconostoc)、乳球菌属(Lactococcus)、双歧杆菌属(Bifidobacterium)、链球菌属(Streptococcus)和肠球菌属(Enterococcus)[9]。产EPS的乳酸菌来源广泛,如食品、婴儿粪便、家禽肠道、水果、人体等。目前已研究报道的产EPS的乳酸菌及其来源见表1。
表1 产胞外多糖的乳酸菌及其来源
Table 1 Extracellular polysaccharides produced by lactic acid bacteria and their sources

乳酸菌名称 来源 参考文献Lactobacillus plantarum KX041 Enterococcus faecium WEFA23 Lactobacillus sp.Ca6 Lactobacillus gasseri FR4 Pediococcus pentosaceus M41 Lactobacillus strains Bacillus tequilensis FR9 Lactobacillus acidophilus 606 Lactobacillus gasser Lactobacillus paracasei M7 Leuconostoc mesenteroides Leuconostoc citreum L3C1E7 Lactobacillus helveticus LZ-R-5 Lactobacillus bulgaricus subsp.delbrueckii中国泡菜曼尼普尔发酵鱼家禽胃肠道鸡海洋鱼干榴莲(属)的果肉家鸡的肠道人类(婴儿)粪便人类阴道母乳传统发酵酵母Pico 奶酪西藏酸乳酒传统保加利亚酸奶[10][11][12][13][14][15][16][17][18][19][20][21][22][23]
1.2 乳酸菌EPS的类型
根据EPS的单糖组成和糖链结构,可将其分为同型多糖(homopolysaccharides,HoPS)与杂型多糖(heteropolysaccharides,HePS)。胞外多糖也可以按照其与细菌位置关系分为粘液多糖与荚膜多糖,粘液多糖被释放到细胞外,荚膜多糖则依附于细胞壁[24]。HoPS是EPS中结构最简单的一类多糖,是由一种单糖重复单位连接形成线性或分枝结构[25]。HoPS 的特点是分子质量大(一般超过106Da)、产量高(高达10 g/L)[26]。能产生HoPS的菌有乳酸菌属(Lactobacillus)、明串珠菌(Leuconostoc)、乳球菌属(Lactococcus)、酒球菌属(Oenococcus)和魏斯氏菌属(Weissella)。HePS是结构复杂的多糖,由不同的单糖重复单位组成,其可能在C2、C3、C4或C6位置分支,也可能没有分支[27]。规则的重复单位包括3~8个单糖(D-葡萄糖、D-半乳糖、L-鼠李糖和其他糖类)、单糖的衍生物(N-乙酰-D-葡萄糖胺、N-乙酰-D-半乳糖胺和葡萄糖醛酸)和非碳水化合物取代基[28]。单糖可以以吡喃糖或呋喃糖形式的α或β异构体形式出现,与HoPS不同,碳水化合物部分是由细胞内糖核苷酸前体合成的[29]。一般来说,HePS的分子质量为104~106Da[30],通常它们的产量为25~600 mg/L[31]。HePS是由乳酸菌属(Lactobacillus)、乳球菌属(Lactococcus)、链球菌属(Streptococcus)和双歧杆菌属(Bifidobacterium)产生的。如不同乳酸菌属(Lactobacillus)产生的HePS的单糖组成存在较大差异,大多由3~6种不同的单糖组成[32]。
目前已有研究报道的乳酸菌EPS很多,如HoPS有右旋糖苷葡、纤维素、果聚糖、菊粉和变形聚糖等;HePS有黄原胶、开菲尔多糖等。常见的HoPS来源与应用见表2。
表2 常见同型多糖生产菌来源及其应用
Table 2 Source and application of common homopolysaccharides produced by bacteria
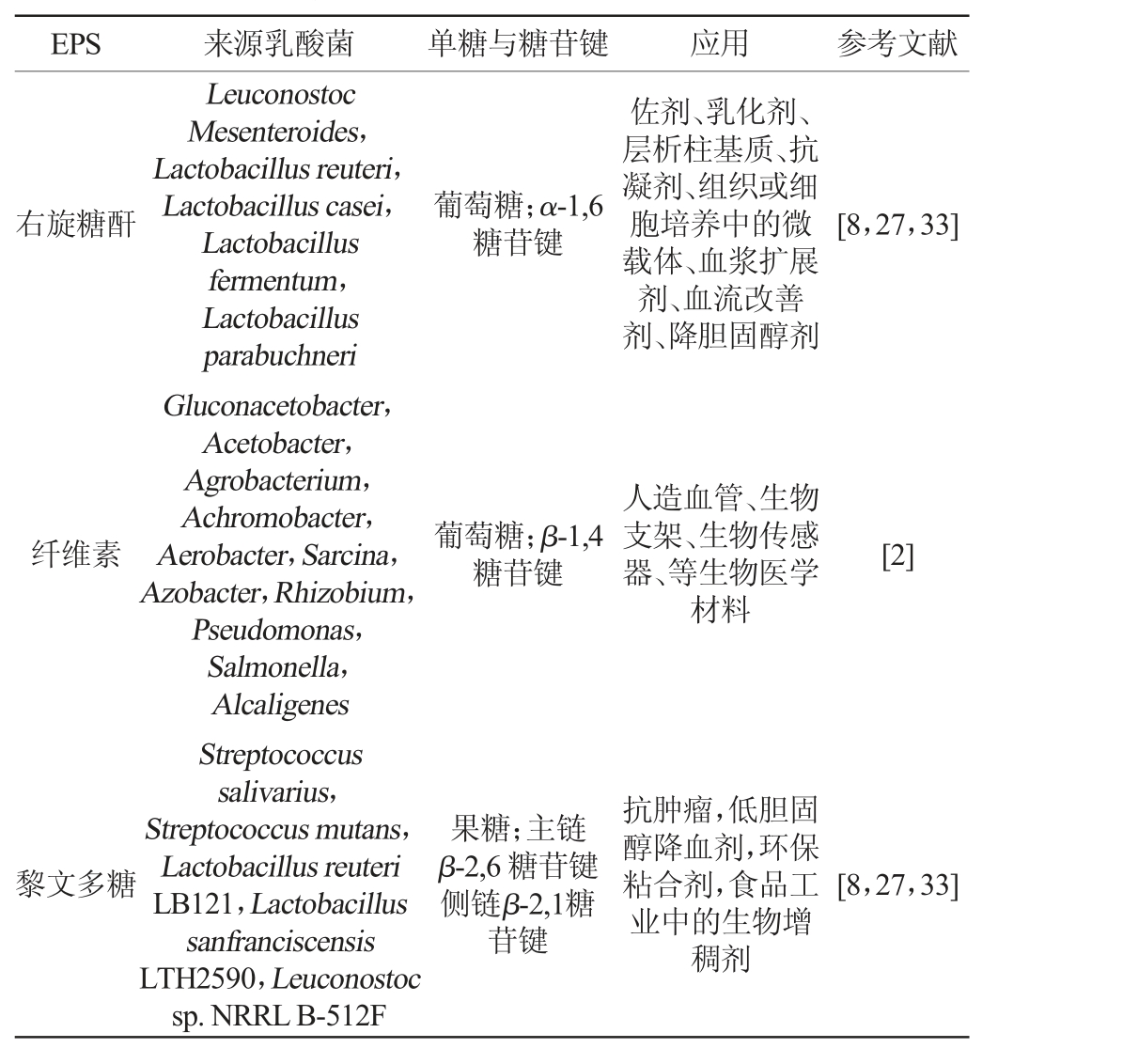
EPS 来源乳酸菌 单糖与糖苷键 应用 参考文献右旋糖酐葡萄糖;α-1,6糖苷键佐剂、乳化剂、层析柱基质、抗凝剂、组织或细胞培养中的微载体、血浆扩展剂、血流改善剂、降胆固醇剂[8,27,33]纤维素葡萄糖;β-1,4糖苷键人造血管、生物支架、生物传感器、等生物医学材料[2]黎文多糖Leuconostoc Mesenteroides,Lactobacillus reuteri,Lactobacillus casei,Lactobacillus fermentum,Lactobacillus parabuchneri Gluconacetobacter,Acetobacter,Agrobacterium,Achromobacter,Aerobacter,Sarcina,Azobacter,Rhizobium,Pseudomonas,Salmonella,Alcaligenes Streptococcus salivarius,Streptococcus mutans,Lactobacillus reuteri LB121,Lactobacillus sanfranciscensis LTH2590,Leuconostoc sp.NRRL B-512F果糖;主链β-2,6 糖苷键侧链β-2,1糖苷键抗肿瘤,低胆固醇降血剂,环保粘合剂,食品工业中的生物增稠剂[8,27,33]
续表
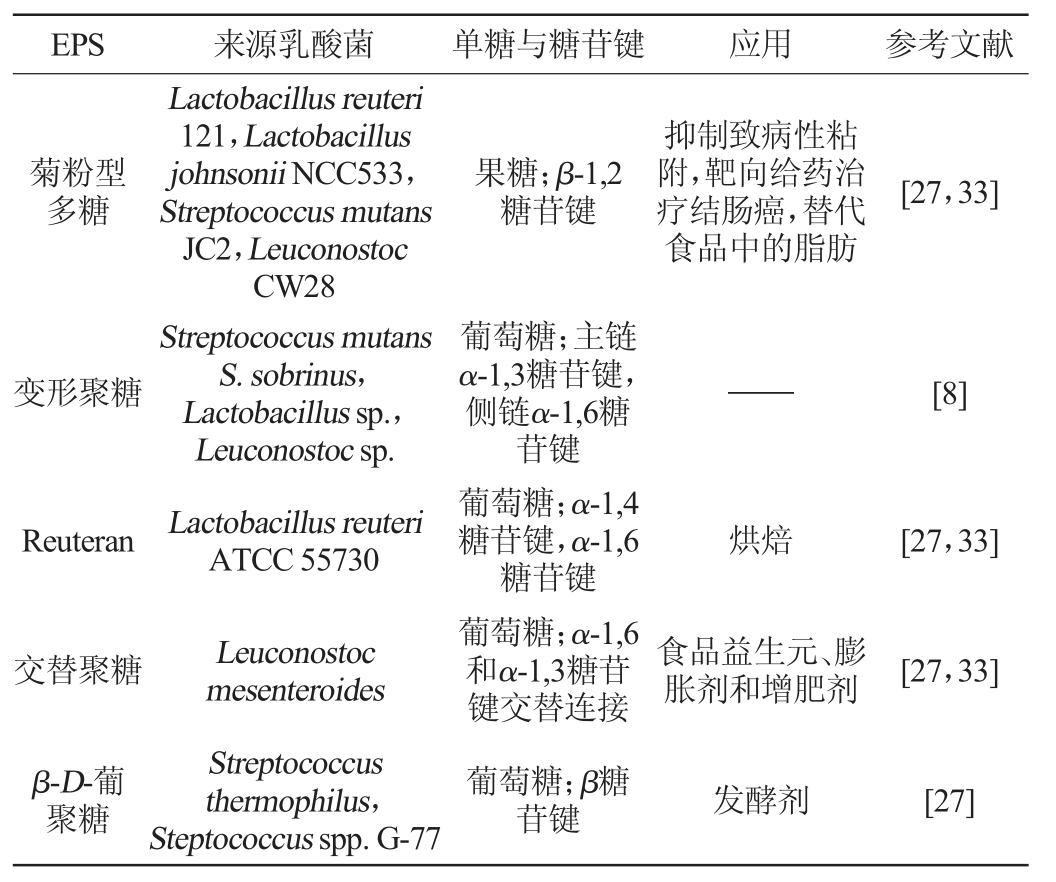
注:“——”表示不明确。
EPS 来源乳酸菌 单糖与糖苷键 应用 参考文献菊粉型多糖果糖;β-1,2糖苷键抑制致病性粘附,靶向给药治疗结肠癌,替代食品中的脂肪[27,33]变形聚糖——[8]Reuteran Lactobacillus reuteri 121,Lactobacillus johnsonii NCC533,Streptococcus mutans JC2,Leuconostoc CW28 Streptococcus mutans S.sobrinus,Lactobacillus sp.,Leuconostoc sp.Lactobacillus reuteri ATCC 55730烘焙[27,33]交替聚糖 食品益生元、膨胀剂和增肥剂[27,33]β-D-葡聚糖Leuconostoc mesenteroides Streptococcus thermophilus,Steptococcus spp.G-77葡萄糖;主链α-1,3糖苷键,侧链α-1,6糖苷键葡萄糖;α-1,4糖苷键,α-1,6糖苷键葡萄糖;α-1,6和α-1,3糖苷键交替连接葡萄糖;β糖苷键发酵剂[27]
1.3 乳酸菌EPS的提取与分离
多糖的提取手段有水提法、酶解提取法、酸碱提取法、超临界提取法、超声波辅助提取法和微波辅助提取法等[34]。但这些提取方法都是针对固体物料。乳酸菌EPS一般存在于溶液体系中,因此通过醇沉的方法就能起到很好的分离效果。乳酸菌发酵完多糖后用离心的方法去除细胞,然后除去蛋白。传统的脱蛋白质方法有Sevage法、三氯乙酸沉淀法、蛋白酶水解法、鞣酸法和盐酸法等[35]。其中Sevage法较为温和,效果好,应用较为广泛。除去蛋白后再用醇沉法得到粗多糖沉淀。粗多糖在经过色谱柱(二乙氨基乙基纤维素或葡聚糖Sephadex色谱柱)分离得到高纯度的EPS[36]。
2 乳酸菌EPS的合成途径
EPS合成有4个途径:Wzx/Wzy依赖途径、ABC转运蛋白依赖途径、合成酶依赖途径和蔗糖酶蛋白胞外合成途径[37]。胞外多糖合成途径见图1[38]。
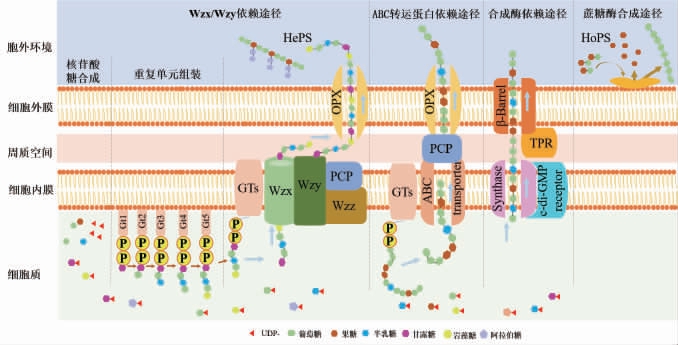
图1 乳酸菌胞外多糖的合成途径
Fig.1 Synthesis pathway of extracellular polysaccharides produced by lactic acid bacteria
Wzx/Wzy依赖途径:Wzx/Wzy依赖通路包括三个阶段:(a)核苷酸糖的合成;(b)重复单元的组装;(c)聚合和输出[39]。首先,糖残基被主动运输到细胞内并转化为各种单体单元,这些单体单元被转移至细胞膜上的脂质载体(C55脂质载体)上并与之连接形成十一异戊烯磷酸(undecaprenyl pyrophosphate,Und-P)[40]。其次,通过GT将更多的糖单元链接至Und-P上产生重复单元,这些重复单元通过Wzx翻转酶在细胞质膜上转移[41]。最后,重复单元通过各种酶修饰后,如甲基化和乙酰化,被Wzy蛋白连接聚合成多糖[42]。组装的多糖通过ABC转运蛋白释放到细胞表面[43]。通过Wzx/Wzy依赖途径合成的多糖主要为HePS[44],如结冷胶和黄原胶[30]。
ABC转运蛋白依赖途径:在ABC转运蛋白依赖的途径中,游离的糖单元被运输到细胞膜上的Und-P上,形成Und-PP-糖分子[45]。位于细胞质侧内膜的特异性GT催化Und-PP-糖分子合成完整的多糖,然后通过位于内膜的外排泵复合物将其转运穿过内膜至细胞表面[46]。ABC转运蛋白依赖途径主要参与荚膜多糖的合成,如黄色粘球菌(Myxococcus xanthus)合成的EPS[45]。
合成酶依赖途径:主要合成的是由同种类型的糖单元组成的HoPS,如海藻酸盐和细菌纤维素[47]。通过合成酶依赖途径,UDP-Glc单元通过膜上的酶(内膜转运体)组装合成BC[48]。
蔗糖酶合成途径:通过蔗糖酶合成途径合成的EPS主要有右旋糖酐和左旋糖酐,其是在细胞外蔗糖酶的作用下,由蔗糖分子裂解获得的前体在细胞外组装产生而成的。蔗糖通过细胞外蔗糖酶转化为细胞膜外的单体单元[47]。根据单糖单元被转移到引物分子的不同,产生果聚糖或葡聚糖。有趣的是,反应条件和蔗糖酶会导致聚糖分子质量改变或分支改变[47]。通过使用不同的引物分子,如麦芽糖、异麦芽糖或黑莓糖等,可以生产更多的低聚糖变体,显示出特定的生物活性的低聚糖,这在生产具有巨大的应用潜力[49]。
3 乳酸菌EPS的活性
乳酸菌EPS具有很多生物活性,如抗氧化、免疫活性、抗肿瘤和抗炎等。这些特性使乳酸菌EPS拥有非常广泛的应用途径与巨大的应用潜力,是材料与药物开发的新型材料。
3.1 抗氧化活性
活性氧簇(reactive oxygen species,ROS)对机体有害,它们很容易与细胞中的其他生物大分子(脂类、蛋白质、脱氧核糖核酸(deoxyribonucleic acid,DNA)和核糖核酸(ribonucleic acid,RNA))发生反应,导致多种疾病产生[50]。从乳酸菌中提取的EPS是天然的抗氧化剂且与合成抗氧化剂相比无毒性作用,是合成抗氧化剂的理想的替代品[51]。格氏乳杆菌(Lactobacillus gasseri)FR4产生的EPS(4 mg/mL)对1,1-二苯基-2-三硝基苯肼(1,1-diphenyl-2-picrylhydrazyl radical,DPPH)(75.95%)有显著的清除活性[13]。从印度蒸米糕中分离的乳明串珠菌(Leuconostoc lactis)KC117496产生的葡聚糖(500 μg/mL)对DPPH自由基(74%)和羟基自由基(97.8%)有显著的清除活性[52]。
3.2 免疫调节活性
据报道,EPS可以作为免疫调节剂调节机体免疫活性[53]。且EPS可以预防免疫缺陷引起的疾病,如炎症性肠病等。XIU L等[54]报道了干酪乳杆菌WXD030产生的EPS能使CD4+T细胞中IFN-γ和IL-4的表达上调,使细胞免疫活性增强。EPS可通过调节细胞因子等信息分子来改变机体免疫功能[55]。如HIDALGO-CANTABRANA C等[53]报道了中性和大分子(分子质量接近106 Da)的EPS能帮助细菌避开宿主的免疫系统,而小分子的EPS能够温和的刺激免疫细胞增强免疫。另外,EPS的支链构象和复杂性对免疫调节活性影响也很大,如,ISMAIL B等[56]研究发现α和β-1,3-键连接的葡萄糖和甘露糖可以激活免疫系统,如促进淋巴细胞增殖。
3.3 抗癌活性
细胞异常发育会导致癌症的产生,对身体各个器官造成损伤。目前治疗癌症的化疗和放疗会给机体带来极大的副作用。开发天然安全、对免疫系统副作用低的抗肿瘤药物是当前研究的热点[17]。乳酸菌EPS独特的分子特性(单糖组成和分子质量等)使其具有良好的抗癌活性,且副作用小[57]。有研究报道,乳糖杆菌产生的EPS G10在400 μg/mL质量浓度下激活和上调了HeLa细胞中Caspase 3蛋白和BAX基因的表达,抑制HeLa细胞的增殖[18]。植物乳杆菌(Lactobacillus plantarum)NCU116产生的EPS通过调节小鼠toll样受体-2(TLR-2)的活性,增加Fas、FasL和c-Jun等促凋亡基因的表达抑制癌细胞的增殖[58]。
3.4 抗炎活性
炎症反应是机体应对各种损伤因子刺激,如细菌、病毒感染等,做出的以防御为主的病理反应。有研究报道乳酸菌EPS具有很好的抗炎活性,如副干酪乳杆菌(Lactobacillus paracasei)和植物乳杆菌(Lactobacillus plantarum)产生的HePS可以激活巨噬细胞的活性,促进巨噬细胞增殖和促炎因子的释放,且与EPS的浓度呈剂量依赖[18]。戊糖乳杆菌(Lactobacillus pentosus)LZ-R-17产生的EPS可促进巨噬细胞分泌炎症因子NO、肿瘤坏死因子-α(tumor necrosis factorα,TNF-α)、白介素(interleukin,II-1β)、IL-6和IL-10[59]。鼠李糖乳杆菌(Lactobacillus rhamnosus)KL37的EPS能刺激小鼠腹腔巨噬细胞分泌促炎因子(TNF-α、IL-6、IL12)和抗炎因子(IL-10)[60]。
3.5 降低胆固醇
胆固醇的积累会导致心血管疾病,危及人们生命安全[61]。降胆固醇药物能控制血清中的胆固醇,但会产生副作用。近年来乳酸菌EPS降低血液胆固醇水平的研究引起了广泛关注。据报道,添加0.1%副干酪乳杆菌(Lactobacillus paracasei)M7产生的EPS可在25 ℃、20 min后将胆固醇(30 μg/mL)溶液中的胆固醇降低至70.78%[19]。从植物乳杆菌(Lactobacillus plantarum)RJF4中提取的EPS可将胆固醇(30 μg/mL)溶液中的胆固醇降低到42.24%[62]。从发酵乳中分离出的粪肠球菌(Enterococcus faecium)F1产生的EPS可将胆固醇(30 μg/mL)溶液中的胆固醇降低48.81%[63]。
3.6 预防糖尿病
糖尿病是由糖代谢异常导致血糖浓度升高危害人体健康的疾病[64]。抗糖尿病药物如阿卡波糖、伏格列糖、米格列醇等会引起腹胀、肠紊乱和腹泻,不适用于胃肠道疾病患者[20]。因此,用EPS替代当前药物治疗具有重要的现实研究意义。有报道从植物乳杆菌(Lactobacillus plantarum)H31中获得的HePS可降低胰岛素抵抗HepG2细胞株中α-淀粉酶的表达,增加GLUT-4、AKT-2和AMPK的基因表达,促进细胞对葡萄糖的吸收[65]。随着EPS H31-2的浓度从10-2降至10-8 mol/L处理胰岛素抵抗的HepG2细胞增加了基因GLUT-4、AMPK和AKT-2的表达,增加葡萄糖消耗,EPS表现出良好的降血糖活性[65]。
3.7 抗病毒活性
病毒性疾病的治疗一般是接种疫苗、化学预防和化疗。除此之外,利用益生菌微生物及其代谢产物对抗病毒是一种新的方法手段。研究表明乳酸菌通过多种机制发挥抗病毒活性,如与病毒直接作用、产生抑制物质或刺激免疫系统对抗病毒[66]。有研究报道德氏乳杆菌(Lactobacillus delbrueckii)OLL1073R-1产生的EPS可上调猪肠上皮细胞IFN-α、IFN-β、MxA和RNase L等干扰素基因的表达,使其抗病毒活性显著提高[67]。由乳酸菌、明串珠菌属和片球菌属所产生的EPS对人类的5型腺病毒具有较强的拮抗活性[68]。植物乳杆菌(Lactobacillus plantarum)产生的EPS能抑制猪睾丸上皮细胞系中的传染性肠胃炎冠状病毒增殖[69]。
3.8 抗生物膜活性
乳酸菌EPS通过减弱细胞表面修饰或减少细胞间的相互作用来抑制细菌的聚集和附着[70]。对由致病菌生物膜引起的传染病有治疗和预防作用。如嗜酸乳杆菌(Lactobacil lus acidophilus)A4来源胞外多糖(released exopolysaccharide,r-EPS)对肠炎沙门氏菌(Salmonella enteritidis),鼠伤寒沙门氏菌(Salmonella typhimurium)KCCM 11806,小肠结肠炎耶氏菌(Yersinia enterocolitica),绿脓杆菌(Pseudomonas aeruginosa)KCCM 11321,李斯特菌(Listeria monocytogenes)ScottA和蜡样芽孢杆菌(Bacillus cereus)均表现出生物膜抑制活性[71]。由罗马尼亚酸奶中融合魏斯氏菌(Weissella confusa)分泌的葡聚糖对白色念珠菌(Monilia albican)SC5314的生物膜抑制达到70%[72]。
4 乳酸菌EPS的应用
乳酸菌EPS具有优良的生物学特性(活性抗氧化活性、免疫活性、抗病毒活性)与理化特性。这些特性使其具有很强应用潜力。在食品加工中,EPS可作为增质剂、稳定剂、增粘剂、生物增稠剂或乳化剂[73]。EPS由于与食物成分相互作用,影响发酵产品的流变学特性和质地[74]。如在酸奶工业中,乳酸菌EPS与蛋白相互作用形成网络结构,改善酸奶粘弹性能,提高保水能力[75]。ANGELIN J等[30]报道称,由明串珠菌属(Leuconostoc)和片球菌属(Pediococcus claussen)株生产的EPS熔点(224 ℃)高,能使乳制品在进行热处理(杀菌)时保持稳定,且热稳定性是乳制品行业的重要特征。
另一方面EPS具有优良的机械强度、生物相容性和生物降解性,可以作为天然的基质材料,开发运用于生物支架、药物包埋等生物医药领域。此外,其还具有生物降解性、生物粘附性、离子、pH值和温度敏感性等众多特性,因此也被广泛应用于药物的靶向递送[76]。如氧化后的右旋糖酐(醛)和聚L-赖氨酸与席夫碱之间作用形成了一种潜在的外科生物粘合剂[77]。WON K K等[78]开发了结冷胶水凝胶包埋软骨细胞,用于软骨再生。GAO F等[79]研究报道,组装的EPS纳米颗粒装载抗癌药物如表阿霉素,在荷瘤小鼠的中增加药物摄入,表现出持续释放模式,使肿瘤体积减少70%。EPS可作为抗原载体或抗原用于制备疫苗[76]。例如,疏水性的羧甲基普鲁兰糖的微粒可有效捕获破伤风类毒素并进行清除[80]。流感嗜血杆菌(Haemophilus influenzae)B型荚膜多糖可以被特异性抗体识别,激活体液免疫产生抗体可以被用作预防儿童各种感染(如脑膜炎)的多价疫苗的重要成分[81]。
在其他方面,对EPS进行修饰,使其带上一些配体基团,应用于医学成像、诊断和治疗等领域。如通过氨基修饰普鲁兰糖可增加肿瘤细胞中的荧光强度[82]。许多基于EPS的多功能显像剂已被开发用于诊断性磁共振(diagnostic magnetic resonance,DMR)、磁共振成像(magnetic resonance imaging,MRI)、正电子发射断层扫描(positron emission to mography,PET)成像,荧光分子断层扫描(fluorescence molecular tomography,FMT)。如超顺磁性氧化铁纳米颗粒与交联葡聚糖涂层(cross-linked dextran coating,CLIO)的结合,这种CLIO可与光敏剂一起用作光动力治疗的治疗剂,可对颈动脉中的动脉粥样硬化细胞进行照射杀伤[83]。
目前部分乳酸菌EPS已经应用于食品、医药、化妆品等行业,且展现出优良的特性,是一种有巨大潜力的新材料。
5 总结与展望
乳酸菌EPS结构丰富,来源广泛,且具有丰富的物理活性和生物活性,能广泛应用于食品,医药化妆品等行业,是一种具有很大潜力的生物材料。乳酸菌EPS的活性与其结构密切相关,如组成EPS的单糖种类、数量与比例,分子质量大小,支链数量,官能团种类等。且乳酸菌EPS的产量受到环境条件(温度和pH)、底物和乳酸菌本身等多种因素的影响。目前EPS的应用主要受限于产量低与产出质量不稳定。随着对EPS生成途径的不断研究,EPS合成途径越来越清晰。这将有利于人为控制EPS的产生,使其大规模应用成为可能。但是,不同的菌株产生的EPS结构不同,其生物学效应也不同,目前发现的乳酸菌EPS的构效关系尚不明确,这些都是新的EPS大规模应用的障碍。因此,需要对EPS结构和其活性进行详细的研究说明,对精准控制产生的EPS的理化性质具有重要意义。同时,开发出有效的方法来获得特定的结构稳定的EPS是非常必要的。上述问题的解决也是未来乳酸菌EPS研究方向及热点,其将促进EPS在很多领域如医疗治疗、食品加工和环境研究等发挥出更大的应用潜力。
[1]刘晶,杨森,陈杨扬,等.副干酪乳杆菌VL8产胞外多糖条件优化及其抗氧化性质[J].中国食品学报,2017,17(5):82-89.
[2]PICHETH G F,PIRICH C L,SIERAKOWSKI M R,et al.Bacterial cellulose in biomedical applications:A review[J].Int J Biol Macromol,2017,104:97-106.
[3] MATSUMURA K,NAKAJIMA N, SUGAI H, et al.Self-degradation of tissue adhesive based on oxidized dextran and poly-L-lysine[J].Carbohydr Polym,2014,113:32-38.
[4]LIU Z,JIAO Y,WANG Y,et al.Polysaccharides-based nanoparticles as drug delivery systems[J].Adv Drug Deliver Rev, 2008, 60(15): 1650-1662.
[5]RESEARCH P M.Global microbial and bacterial cellulose market 2019-by manufacturers, regions, type, application, sales, revenue, and forecast to 2025[EB/OL].[9/26].https://www.promarketresearch.com/global-microbial-and-bacterial-cellulose-market-2018-by-25638.html.
[6] ABARQUERO D,RENES E,FRESNO J M,et al.Study of exopolysaccharides from lactic acid bacteria and their industrial applications:a review[J].Int J Food Sci Tech,2022,57(1):16-26.
[7]YALDA R S,AHMAD Y K,BAHRAM P G.A comprehensive review of anticancer,immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides[J].Carbohydr Poly,2019,217:79-89.
[8]YILDIZ H,KARATAS N.Microbial exopolysaccharides: Resources and bioactive properties[J].Process Biochem,2018,72:41-46.
[9] SANALIBABA P, CAKMAK G A.Exopolysaccharides production by lactic acid bacteria[J].Appl Microbiol,2016,2(2):1000115.
[10] YUANMEI X, YANLONG C, XIN W, et al.Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041[J].Int J Biol Macromol,2019,128:117-130.
[11]JIA K Y,TAO X Y,LIU Z Q,et al.Characterization of novel exopolysaccharide of Enterococcus faecium WEFA23 from infant and demonstration of its in vitro biological properties[J].Int J Biol Macromol,2019,128:710-717.
[12]TRABELSI I,SLIMA S B,CHAABANE H,et al.Purification and characterization of a novel exopolysaccharides produced by Lactobacillus sp.Ca6[J].Int J Biol Macromol,2015,74:541-546.
[13]RIZWANA P R,MARIMUTHU A,ABRAHAM D R.Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties[J].Int J Biol Macromol,2018,109:772-783.
[14] AYYASH M, ABU-JDAYIL B, OLAIMAT A, et al.Physicochemical,bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41[J].Carbohydr Polym,2020,229:115462.
[15]KHALIL E,ABD MANAP M,MUSTAFA S,et al.Probiotic properties of exopolysaccharide-producing Lactobacillus strains isolated from tempoyak[J].Molecules,2018,23(2):398.
[16]RANI R P,ANANDHARAJ M,SABHAPATHY P,et al.Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken[J].Int J Biol Macromol,2017,96:1-10.
[17]ABDELGHANY K,HAMOUDA R,ABD ELHAFEZ E,et al.A potential role of Lactobacillus acidophilus LA1 and its exopolysaccharides on cancer cells in male albino mice[J].Biotechnol Biotec Eq,2015,29(5):977-983.
[18]SUNGUR T,ASLIM B,KARAASLAN C,et al.Impact of exopolysaccharides(EPSs)of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells(HeLa)[J].Anaerobe,2017,47:137-144.
[19] BILQEESA B, BIJENDER K B.Hypocholesterolemic potential and bioactivity spectrum of an exopolysaccharide from a probiotic isolate Lactobacillus paracasei M7[J].Bioact Carbohydr Diet Fibre,2019,19:100191.
[20]OSMAN T,MUSTAFA T Y,ENES D.Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities[J].Int J Biol Macromol,2019,136:436-444.
[21]DOMINGOS LOPES M F P,LAMOSA P,STANTON C,et al.Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese[J].Lett Appl Microbiol,2018,67(6):570-578.
[22]YOU X,LI Z,MA K,et al.Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5[J].Carbohyd Polym,2020,235:115977.
[23]MAKINO S,SATO A,GOTO A,et al.Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp.bulgaricus OLL1073R-1[J].J Dairy Sci,2016,99(2):915-923.
[24]JOLLY L,VINCENT S J F,DUBOC P,et al.Exploiting exopolysaccharides from lactic acid bacteria[J].Anton Leeuw,2002,82(1-4):367-374.
[25]NGUYEN P,NGUYEN T,BUI D,et al.Exopolysaccharide production by lactic acid bacteria: the manipulation of environmental stresses for industrial applications[J].AIMS Microbiol,2020,6(4):451-569.
[26] KIERAN M L, EMANUELE Z, AIDAN C, et al.Lactic acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization,and health benefits[J].Annu Rev Food Sci Technol,2018,9(1):155-176.
[27]ZANNINI E,WATERS D M,COFFEY A,et al.Production,properties,and industrial food application of lactic acid bacteria-derived exopolysaccharides[J].Appl Microbiol Biot,2016,100(3):1121-1135.
[28] SUSANN M, HARALD R, DORIS J.Influence of exopolysaccharides on the structure,texture,stability and sensory properties of yoghurt and related products[J].Int Dairy J,2016,52:57-71.
[29]BAJPAI V K,MAJUMDER R,RATHER I A,et al.Extraction,isolation and purification of exopolysaccharide from lactic acid bacteria using ethanol precipitation method[J].Bangl J Pharmacol,2016,11(3):573-576.
[30]ANGELIN J,KAVITHA M.Exopolysaccharides from probiotic bacteria and their health potential[J].Int J Biol Macromol,2020,162:853-865.
[31]LEROY F,DE VUYST L.Advances in production and simplified methods for recovery and quantification of exopolysaccharides for applications in food and health[J].J Dairy Sci,2016,99(4):3229-3238.
[32] SUZUKI C, KOBAYASHI M, KIMOTO-NIRA H.Novel Exopolysaccharides produced by Lactococcus lactis subsp. lactis, and the diversity of epsE genes in the exopolysaccharide biosynthesis gene clusters[J].Biosci Biotech Bioch,2014,77(10):2013-2018.
[33] PATEL S, MAJUMDER A, GOYAL A.Potentials of exopolysaccharides from lactic acid bacteria[J].Ind J Microbiol,2012,52(1):3-12.
[34]徐涵,刘云,阚欢.天然多糖提取纯化及生理功能活性研究进展[J].食品安全质量检测学报,2022,13(5):1382-1390.
[35]王珊,黄胜阳.植物多糖提取液脱蛋白方法的研究进展[J].食品科技,2012,37(9):188-191.
[36]邸维,张英春,易华西,等.乳酸菌胞外多糖结构解析的研究方法[J].分析化学,2018,46(6):875-882.
[37]SCHMID J,SIEBER V,REHM B.Bacterial exopolysaccharides:biosynthesis pathways and engineering strategies[J].Front Microbiol, 2015,6:496.
[38]MOHD NADZIR M,NURHAYATI R W,IDRIS F N,et al.Biomedical applications of bacterial exopolysaccharides: A review[J].Polymers,2021,13(4):530.
[39]LUC D V,FILIP D V,FREDERIK V,et al.Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria[J].Int Dairy J,2001,11(9):687-770.
[40] REHM B H A.Bacterial polymers: biosynthesis,modifications and applications[J].Nat Rev Microbiol,2010,8(8):578-592.
[41]SONALI R,LATA S B U.Microbial exopolysaccharides:Synthesis pathways,types and their commercial applications[J].Int J Biol Macromol,2020,157:577-583.
[42]ISLAM S T,LAM J S.Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway[J].Can J Microbiol,2014,60(11):697-716.
[43] DE VUYST L, DEGEEST B.Heteropolysaccharides from lactic acid bacteria[J].FEMS Microbiol Rev,1999,23(2):153-177.
[44]YILMAZ T,S,IMS,EK Ö.Potential health benefits of ropy exopolysaccharides produced by Lactobacillus plantarum[J].Molecules,2020,25(14):3293.
[45]PÉREZ-BURGOS M,GARCÍA-ROMERO I,JUNG J,et al.Characterization of the exopolysaccharide biosynthesis pathway in Myxococcus xanthus[J].J Bacteriol,2020,202(19):243-252.
[46]CUTHBERTSON L,KOS V,WHITFIELD C.ABC Transporters involved in export of cell surface glycoconjugates[J].Microbiol Mol Biol R,2010,74(3):341-362.
[47]UA-ARAK T,JAKOB F,VOGEL R F.Fermentation pH modulates the size distributions and functional properties of Gluconobacter albidus TMW 2.1191 levan[J].Front Microbiol,2017,8:807.
[48] WANG S, HAN Y, CHEN J, et al.Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp.W1[J].Polymers,2018,10(9):963.
[49]HU Y,WINTER V,CHEN X Y,et al.Effect of acceptor carbohydrates on oligosaccharide and polysaccharide synthesis by dextransucrase DsrM from Weissella cibaria[J].Food Res Int,2017,99:603-611.
[50] RAHBAR S Y, YARI K A, POURGHASSEM G B.A comprehensive review of anticancer, immuno-modulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides[J].Carbohydr Polym,2019,217:79-89.
[51] ADESULU-DAHUNSI A T, SANNI A I, JEYARAM K.Production,characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44[J].LWT-Food Sci Technol, 2018, 87:432-442.
[52]SARAVANAN C,KAVITAKE D,KANDASAMY S,et al.Production,partial characterization and antioxidant properties of exopolysaccharide α-D-glucan produced by Leuconostoc lactis KC117496 isolated from an idli batter[J].J Food Sci Technol,2019,56(1):159-166.
[53]HIDALGO-CANTABRANA C,LÓPEZ P,GUEIMONDE M,et al.Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and Bifidobacteria[J].Probiotics Antimicrobial Proteins,2012,4(4):227-237.
[54]XIU L,ZHANG H,HU Z,et al.Immunostimulatory activity of exopolysaccharides from probiotic Lactobacillus casei WXD030 strain as a novel adjuvant in vitro and in vivo[J].Food Agr Immunol,2018,29(1):1086-1105.
[55] SINGH P, SAINI P.Food and health potentials of exopolysaccharides derived from Lactobacilli[J].Microbiol Res J Int,2017,22(2):1-14.
[56]ISMAIL B,NAMPOOTHIRI K M.Exposition of antitumour activity of a chemically characterized exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510[J].Biologia,2013,68(6):1041-1047.
[57]WANG K,LI W,RUI X,et al.Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810[J].Int J Biol Macromol,2014,63:133-139.
[58]ZHOU X,HONG T,YU Q,et al.Exopolysaccharides from Lactobacillus plantarum NCU116 induce c-Jun dependent Fas/Fasl-mediated apoptosis via TLR2 in mouse intestinal epithelial cancer cells[J].Sci Rep,2017,7(1):110226.
[59]YOU X,YANG L,ZHAO X,et al.Isolation,purification,characterization and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan kefir[J].Int J Biol Macromol,2020,158:408-419.
[60]CISZEK-LENDA M,NOWAK B,SROTTEK M,et al.Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37:effects on the production of inflammatory mediators by mouse macrophages[J].Int J Exp Pathol,2011,92(6):382-391.
[61]MAJEED M,MAJEED S,NAGABHUSHANAM K,et al.Evaluation of thein vitro cholesterol-lowering activity of the probiotic strain Bacillus coagulans MTCC 5856[J].Int J Food Sci Tech,2019,54(1):212-220.
[62]DILNA S V,SURYA H,ASWATHY R G,et al.Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4[J].LWT-Food Sci Technol,2015,64(2):1179-1186.
[63] BHAT B, BAJAJ B K.Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei[J].Bioresource Technol,2018,254:264-267.
[64]XIE W,ZHANG Y,WANG N,et al.Novel effects of macrostemonoside A,a compound from Allium macrostemon Bung,on hyperglycemia,hyperlipidemia,and visceral obesity in high-fat diet-fed C57BL/6 mice[J].Eur J Pharmacol,2008,599(1-3):159-165.
[65] HUANG Z H, LIN F X, ZHU X Y, et al.An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway[J].Int J Biol Macromol,2020,143(C):775-784.
[66]AL KASSAA I,HOBER D,HAMZE M,et al.Antiviral potential of lactic acid bacteria and their bacteriocins[J].Probiotics Antimicrobial Proteins,2014,6(3-4):177-185.
[67] KANMANI P, ALBARRACIN L, KOBAYASHI H, et al.Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells[J].Mol Immunol,2018,93:253-265.
[68]BILIAVSKA L,PANKIVSKA Y,POVNITSA O,et al.Antiviral activity of exopolysaccharides produced by lactic acid bacteria of the genera Pediococcus, Leuconostoc and Lactobacillus against human adenovirus type 5[J].Medicina,2019,55(9):519.
[69] YANG Y, SONG H, WANG L, et al.Antiviral effects of a probiotic metabolic products against transmissible gastroenteritis Coronavirus[J].J Prob Health,2017,5(3):799-807.
[70] KANMANI P,SUGANYA K,KUMAR R S, et al.Synthesis and functional characterization of antibiofilm exopolysaccharide produced by Enterococcus faecium MC13 isolated from the gut of fish[J].Appl Biochem Biotech,2013,169(3):1001-1015.
[71]KIM Y,OH S,KIM S H.Released exopolysaccharide(r-EPS)produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7[J].Biochem Bioph Res Co,2009,379(2):324-329.
[72] ROSCA I, PETROVICI A R, PEPTANARIU D, et al.Biosynthesis of dextran by Weissella confusa and its in vitro functional characteristics[J].Int J Biol Macromol,2018,107:1765-1772.
[73] KORCZ E, VARGA L.Exopolysaccharides from lactic acid bacteria:Techno-functional application in the food industry[J].Trends Food Sci Tech,2021,110:375-384.
[74] KENZA Z, Ma G L, ALICIA P, et al.Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria[J].Carbohydr Polym,2017,174:646-657.
[75] ZEIDAN A A, POULSEN V K, JANZEN T, et al.Polysaccharide production by lactic acid bacteria: from genes to industrial applications[J].FEMS Microbiol Rev,2017,41:168-200.
[76] MOSCOVICI M.Present and future medical applications of microbial exopolysaccharides[J].Front Microbiol,2015,6:1012.
[77]MATSUMURA K,NAKAJIMA N,SUGAI H,et al.Self-degradation of tissue adhesive based on oxidized dextran and poly-L-lysine[J].Carbohydr Polym,2014,113:32-38.
[78] WON K K,JOO H C,MYEONG E S,et al.Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel[J].Int J Biol Macromol,2019,141:51-59.
[79]GAO F,LI L,ZHANG H,et al.Deoxycholic acid modified-carboxymethyl curdlan conjugate as a novel carrier of epirubicin: In vitro and in vivo studies[J].Int J Pharmaceut,2010,392(1-2):254-260.
[80]MOCANU G,MIHAÏ D,DULONG V,et al.Anionic polysaccharide hydrogels with thermosensitive properties[J].Carbohydr Polym, 2011,83(1):52-59.
[81] ALBANI S M F, DA SILVA M R, FRATELLI F, et al.Polysaccharide purification from Haemophilus influenzae type b through tangential microfiltration[J].Carbohydr Polym,2015,116:67-73.
[82]PRAJAPATI V D,JANI G K,KHANDA S M.Pullulan:An exopolysaccharide and its various applications[J].Carbohydr Polym,2013,95(1):540-549.
[83] TASSA C, SHAW S Y, WEISSLEDER R.Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging,molecular diagnostics,and therapy[J].Acc Chem Res, 2011, 44(10):842-852.