乳酸菌是能利用糖类发酵而产生大量乳酸的一类革兰氏阳性细菌的总称,在人体、动植物体及发酵食品中广泛存在[1-2]。在乳制品的发酵过程中,乳酸菌是一种重要的发酵剂,它们在发酵过程中可将可溶性碳水化合物转化为乳酸,能够调节食品的酸碱度,延缓食品腐败,增加食品的保质期。乳酸菌也会参与发酵过程中的代谢反应,可产生一系列风味化合物,赋予酸奶酸味和其他丰富的口感。另外,在发酵过程中,乳酸菌还会分解食材中的一些复杂物质,使其能够被人体更易消化和吸收[3-5]。因此,乳酸菌被广泛应用于乳制品工业中。随着研究人员对乳酸菌的关注,其所具有的生物活性因子发挥出的抗氧化[6-7]、抗肿瘤[8-9]、抗菌[10]、免疫调节[11-12]、降血压[13]、抗食物过敏[14-15]等特性也逐渐被发现,并得到广泛肯定和应用[16-17]。近年来,关于乳酸菌的生物活性研究主要集中在抗氧化、抗肿瘤、抗病毒几方面,而对抗食物过敏的研究较少。此外,食物过敏的患病率越来越高,尤其是儿童患者,乳酸菌已被证实可有效治疗食物过敏且不会使机体出现不良反应,但关于乳酸菌抗食物过敏的作用机制尚未研究清楚。该文阐述了乳酸菌抗食物过敏和免疫调节特性的作用机制,并对其黏附作用进行了综述,旨在为乳酸菌的开发利用提供一定的理论参考。
1 乳酸菌的抗食物过敏特性
食物过敏是指对特定过敏原敏感的机体接触到特定食物抗原时引发的异常免疫反应[18-19]。最常见的食物过敏反应是免疫球蛋白E(immunoglobulin E,IgE)介导的,症状从轻度瘙痒、胃疼和皮疹到严重过敏反应不等,另外,食物过敏也会造成机体器官损伤,主要包括皮肤、呼吸道、消化道、心血管及神经系统[20-22]。近些年来,食物过敏的发生率越来越高,逐渐成为一种重大的公共卫生问题,但仍没有开发出有效的治疗策略。肠道菌群的深入研究凸显了黏膜免疫系统对食物抗原免疫适应的重要机制,益生菌也由于其具有调节肠道微生物菌群的功能而被认为是有希望预防食物过敏的靶点[23]。目前,已有学者对乳酸菌的抗食物过敏特性进行了研究。NODAM等[24]报道了口服副干酪乳杆菌(Lactobacillus paracasei)IJH-SONE68所产胞外多糖对速发型过敏模型小鼠的症状起到改善作用。乳酸杆菌(Lactobacillus)CJLP133和CJLP243可通过抑制致敏小鼠脾细胞分泌白细胞介素(interleukin,IL)-4、IL-5、IL-13等细胞因子以及小肠中辅助性T细胞2型(Th2)相关基因产物如转录因子GATA3的表达方式来调节食物过敏小鼠的免疫反应,进而缓解小鼠的过敏症状[25]。
1.1 乳酸菌的抗食物过敏作用机制
食物过敏是Th2细胞依赖型免疫反应,其特征是通过产生细胞因子IL-4、IL-5和IL-13,促进B细胞转化为浆细胞,产生抗体IgE,IgE结合Fcε效应细胞上的受体,如肥大细胞,这些细胞会导致其他化学介质的产生[26-28]。另外,在非易感个体中,辅助性T细胞1型(Th1细胞)反应伴随着干扰素γ(interferon,IFN-γ)和IL-2的产生,以及IgE水平的降低[29]。另外,Th17和调节性T细胞(Treg)的失衡也会引起机体的免疫失调[30]。食物过敏具体的发病机制见图1。
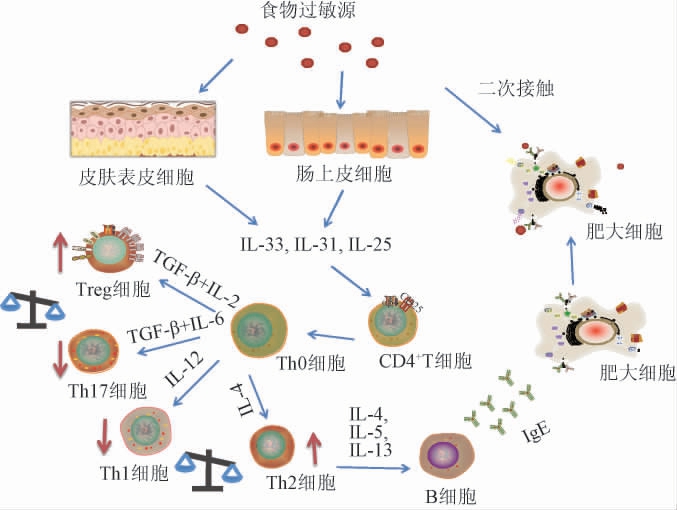
图1 食物过敏发病机制
Fig.1 Pathogenesis of food allergy
乳酸菌的抗食物过敏作用机制主要有以下几方面:
(1)免疫调节反应
大多数食物过敏性疾病反映了淋巴细胞调控的免疫失衡,尤其是胃肠道中调节性T细胞的异常功能,而发酵乳制品的饮食促进了免疫调节乳酸菌与肠道相关淋巴组织系统细胞的相互作用[31-34]。因此,可通过乳酸菌的免疫调节特性改变机体内细胞因子的含量进而发挥出抗食物过敏特性。研究表明,口服德氏乳杆菌(Lactobacillus delbrueckii)UFA-H2b20可刺激用卵清蛋白诱导的过敏性气道炎症小鼠的免疫系统,减少嗜酸性粒细胞和肺泡巨噬细胞的数量以及IgE水平,改善呼吸参数并抑制肺部的炎症反应[35]。SHIN H S等[18]研究了益生菌混合物P5(乳酸乳球菌(Lactococcus lactis)KF140、戊糖片球菌KF159、戊糖乳杆菌(Pediococcus pentosaceus)KF340、副干酪乳杆菌698和解淀粉芽孢杆菌(Bacillus amyloliquefaciens)26N)对卵清蛋白诱导的食物过敏小鼠的缓解作用。结果表明,服用P5可通过抑制IgE、Th2和Th17细胞因子的分泌,并诱导CD4+Foxp3+调节性T细胞的生成达到显著抑制过敏反应和降低直肠温度的效果。LOKE P等[36]通过添加益生菌佐剂研究其是否提高了花生口服免疫疗法(oral immunotherapy,OIT)治疗过敏的有效性或安全性,结果表明,向花生OIT中添加益生菌佐剂并没有提高OIT疗法的有效性,但益生菌佐剂可通过减少胃肠道症状和全身反应的负担,体现出有意义的安全性益处,特别是对1~5岁的儿童。机体在外部接触或内部食用过敏原后,会诱导屏障部位稳态的扰动,可以激活或降低免疫系统的阈值,进而出现过敏症状。因此,食物过敏源于免疫耐受机制的缺陷,通过乳酸菌的免疫调节特性抑制Th2细胞因子的分泌及IgE的产生,进而达到平衡机体的免疫系统作用,可有效的缓解过敏。
(2)调节肠道微生物
食物过敏通常会引起机体肠道微生物紊乱进而使肠道免疫屏障发生功能障碍[37-38]。并且,肠道微生物的数量或多样性也会通过微生物分子与免疫细胞的模式识别受体的相互作用而对口服耐受性产生影响[39-42]。已有研究证实,肠道微生物菌群在调节对食物过敏的易感性方面起着关键作用,通过益生菌调节肠道微生物菌群的组成或功能已被认为是防止食物过敏的新希望[43]。DUAN C等[44]对植物乳杆菌(Lactobacillus plantarum)JC7的抗过敏作用进行了研究,结果表明,植物乳杆菌JC7可增加卵清蛋白(ovalbumin,OVA)致敏小鼠盲肠中微生物菌群的丰富度、多样性和均匀性,使盲肠中拟杆菌门丰度提高,厚壁菌门丰度降低,即通过口服植物乳杆菌JC7可以恢复致敏小鼠紊乱的肠道微生物菌群。也有研究表明,鼠李糖乳杆菌(Lactobacillus rhamnosus)GG可使过敏小鼠肠道内拟杆菌类相对丰度增加,组胺、苯丙氨酸、色氨酸、花生四希酸等肠道内容物代谢产物显著降低,从而缓解小鼠过敏症状[45]。CUKROWSKA B等[46]评估了含有鼠李糖乳杆菌OCK 0900、鼠李糖乳杆菌OCK 0908和干酪乳杆菌(Lacticaseibacillus casei)OCK 0918的益生菌制剂对患有牛奶蛋白过敏症的2岁以下儿童的有效性,发现OCK菌株补充剂改变了幼儿的肠道微生物菌群结构,在接受了OCK菌株混合物的实验组的肠道内显示出具有较高丰度的拟杆菌属,并有效的改善了食物过敏的严重程度。由以上研究表明,改变肠道微生物组的种类、数量与食物过敏风险之间存在关联。通过乳酸菌改变机体肠道微生物菌群的丰富度、种类,恢复由过敏引起的肠道微生物紊乱来治疗食物过敏是一种较为安全且高效的途径。
(3)调节信号通路
有研究表明,食物过敏过程可能伴随着蛋白酶体和NOD样受体信号通路的激活,容易引起炎症因子的释放和前列腺等疾病的发生[47]。乳酸菌可通过调节机体免疫系统信号通路来缓解食物过敏症状。LI N等[48]建立了由OVA诱导的食物过敏小鼠模型,对短双歧杆菌(Bifidobacterium)M-16V的抗食物过敏特性进行研究。结果表明,短双歧杆菌M-16V可通过改变肠道微生物群、调节Th1/Th2比例失衡的IL-33/ST2的信号通路来缓解OVA诱导的食物过敏症状。TIAN X等[49]研究表明动物双歧杆菌(Bifidobacterium animalis)KV9和阴道乳杆菌(Lactobacillus vaginalis)FN3通过激活Toll样受体(toll-like receptor,TLR)4通路,调节TLR-1和TLR-4的表达,实现Th1/Th2细胞免疫平衡,表明两株菌株具有抗过敏活性。细胞代谢物不仅是为生物过程提供底物,还通过对代谢途径的影响或调节其他调节蛋白提供关键信号,并通过信号通路将信号传导至代谢以外的过程,包括免疫激活和细胞因子分泌。因此,乳酸菌通过相关信号通路激活机体免疫系统并抑制Th2细胞因子的分泌,调整Th1/Th2比例失衡,进而达到缓解过敏症状。
1.2 活性/非活性乳酸菌菌体抗过敏活性比较
益生菌特别是乳酸菌被认为是“活的微生物”,因此大多数研究人员在探讨乳酸菌的抗食物过敏特性时经常通过活菌株的形式定植在宿主体内成为肠道菌群,从而缓解一些过敏症状[50-51]。然而乳酸菌的菌体细胞还包括热致死菌体细胞和冻干菌体细胞等[52]。在发酵乳制品中,是否只有活菌体细胞具有抗食物过敏特性一直是争论的焦点。一些研究表明,非存活的乳酸菌也可以显示出免疫调节活性,如用加热杀死的乳酸杆菌GG细胞制剂灌胃食物过敏小鼠时,小鼠脾细胞分泌的IL-6水平显著增加,这些结果表明热致死乳杆菌GG可以以佐剂样的方式增强宿主动物的系统和粘膜免疫反应,并使粪便中抗原特异性IgA含量增加[53]。NONAKA Y等[54]对植物乳杆菌S-PT84其进行热致死处理,测定了热致死菌株对OVA诱导的BALB/c致敏小鼠的抗过敏活性,结果表明灌胃S-PT84可降低OVA诱导的过敏小鼠血清IgE水平,并通过调节Th1/Th2平衡和诱导调节性T细胞显示出抗过敏作用。出现这种现象的原因可能是由热致死菌体制备的乳酸菌的细胞壁和细胞质等成分也具有抗食物过敏特性。因此,研究者们在探讨乳酸菌的抗食物过敏特性时也可考虑对其不同的形式进行研究,筛选出乳酸菌抗食物过敏特性最有效的形式。
2 乳酸菌的免疫调节特性
益生菌对过敏症状的潜在预防和治疗作用主要是通过调节Th1/Th2免疫平衡,以及促进免疫耐受性来正向调节免疫应答[55]。因此,乳酸菌的抗食物过敏特性与其免疫调节特性是密切相关的。免疫调节是指免疫系统中的免疫细胞和免疫分子与其他系统之间相互作用,使得免疫应答水平处于一种稳定的状态[56-57]。目前已有研究者对乳酸菌的免疫调节特性进行了探讨,ZHANG N等[58]研究了从母乳喂养婴儿粪便中分离出的植物乳杆菌(Lactobacillus plantarum)BF-15对免疫调节和肠道微生物群失调的影响,结果表明菌株BF-15可以保护模型小鼠免受胸腺和脾脏指数、脾淋巴细胞增殖、血清溶血素抗体水平和巨噬细胞吞噬指数等多种免疫相关指标的降低。还有研究发现在用卷曲乳杆菌(Lactobacillus crispatus)处理的肠道上皮细胞VK2/E6E7细胞中,炎症因子IL-2、IL-6和IL-17的浓度显著上调,IL-8的浓度显著下调。表明卷曲乳杆菌可有效保护细胞免受白色念珠菌菌丝和孢子的毒力影响[59]。
2.1 乳酸菌免疫调节作用机制
乳酸菌通过不同的信号通路及机制发送信号激活机体的免疫细胞,并调节机体免疫相关基因表达发挥积极的免疫调节作用[60-61]。MIN F等[62]探讨了从婴儿粪便中分离出的干酪乳杆菌(Lacticaseibacillus casei)NCU011054对环磷酰胺诱导的免疫抑制小鼠免疫应答和肠道菌群的影响,结果表明干酪乳杆菌NCU0110541可提高粘蛋白(Muc2)和紧密连接蛋白(ZO-1、occludin和claudin-1)的水平,且可上调两种转录因子(T-bet和GATA-3)信使核糖核酸(messenger ribonucleic acid,mRNA)水平和CD4+T细胞的数量,并通过TLR/核转录因子(nuclear transcription factor,NF)-κB途径调节Th1/Th2平衡。LEE J等[63]研究发现罗伊氏乳杆菌(Lactobacillus reuteri)MG5462、乳酸杆菌MG4668、MG5278以及发酵乳杆菌(Lactobacillusfermentum)MG4263、MG4268、MG4282的无细胞上清液(cell-free supernatant,CFS)可通过激活NF-κB和MAPK信号通路显著增加了巨噬细胞的吞噬作用,并增强了促炎因子如肿瘤坏死因子(tumor necrosis factor,TNF)-α、IL-6的水平,同时上调了诱导型一氧化氮合成酶(inducible nitric oxide synthase,iNOS)和环氧化酶-2(cyclooxygenase-2,COX-2)的蛋白表达,增强了巨噬细胞的免疫调节活性。乳酸菌通过信号通路发送传导信号进而控制免疫系统中各种免疫细胞的活化、增殖和凋亡,以及各种细胞因子的含量变化或者参与免疫调节特定分子的激活和表达,发挥出乳酸菌的免疫调节特性。
另外,巨噬细胞可通过吞噬作用清除病原体,同时起到部分的抗原递呈作用,是机体免疫系统中很重要的一种免疫细胞[64]。因此,研究者们通过乳酸菌对巨噬细胞的影响来验证乳酸菌的免疫调节特性,如MATSUBARA V H等[65]研究发现,乳酸杆菌(鼠李糖乳杆菌LR32、干酪乳杆菌L324m或嗜酸乳杆菌(Lactobacillus acidophilus)NCFM)预处理的巨噬细胞上dectin-1的表达及TLR4水平降低,TLR2mRNA水平、IL-10和IL-1β水平升高。并且,乳杆菌损坏了巨噬细胞对病原体模式识别分子的识别,改变了促炎因子的产生,从而调节炎症。JIA D J等[66]从葡聚糖磷酸钠诱导的结肠炎小鼠中筛选出一株新的益生菌菌株——约氏乳杆菌(Lactobacillus reuteri)。在巨噬细胞缺失的小鼠模型中,灌胃约氏乳杆菌可通过特异性提高小鼠肠道巨噬细胞比例和IL-10分泌来缓解结肠炎。另外,临床试验发现,约氏乳杆菌可激活天然巨噬细胞转化为CD206巨噬细胞,并通过TLR1/2-STAT3通路释放IL-10,缓解结肠炎。乳酸菌通过刺激巨噬细胞增强其活性,可以吞噬和消化多种病原微生物,还可将抗原性的物质吞噬后递呈给Th细胞识别,使免疫系统中细胞因子达到平衡,从而达到免疫调节的效果[67-68]。
还有些乳酸杆菌作为益生菌制剂,可通过其黏附能力黏附在肠上皮细胞上并定植于肠道中与肠道细胞进行信号交流,进而增强宿主的免疫机能[69-70]。乳酸菌定植肠道后发挥免疫调节作用的途径主要包括抑制肠上皮细胞凋亡,与病原菌竞争肠粘膜的黏附及定植位点[71-73]。YAN F等[74]研究发现,从鼠李糖乳杆菌GG中纯化出的P75和P40两个新的蛋白都能激活蛋白激酶B(Akt),从而抑制细胞因子诱导的肠上皮细胞凋亡,并促进了人和小鼠结肠上皮细胞以及培养的小鼠结肠移植物的细胞生长。乳酸菌在肠道菌群中发挥调节作用的机制示意图见图2。肠道上皮屏障功能可保护宿主免受病原体感染,乳杆菌可利用其表面成分和代谢产物诱导粘蛋白表达,增强肠上皮紧密连接,保护肠上皮细胞,从而加强肠上皮屏障功能,进而达到免疫调节的特性[75]。
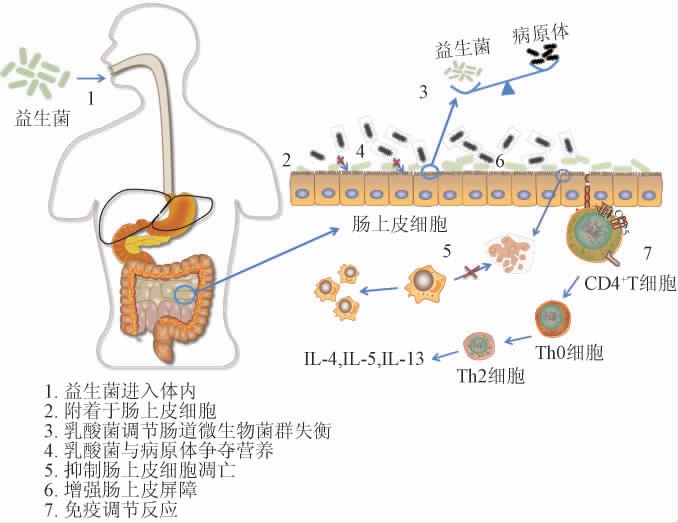
图2 乳酸菌在肠道菌群中的潜在作用机制示意图
Fig.2 Schematic diagram of the potential mechanism of lactic acid bacteria in the intestinal flora
2.2 乳酸菌的黏附作用
乳酸菌之所以能够定植于机体肠道,主要是由于其所具有的黏附作用。黏附通常是指微生物与机体的肠上皮细胞接触后,通过生物化学作用与机体的肠上皮细胞相连接[76]。乳酸菌通过黏附作用定植于上皮细胞后可发挥维持肠道菌群稳态、调控粘蛋白表达、加强细胞间信号交流的作用,为乳酸菌对机体进行免疫调节提供了作用途径[77-79]。并且,一些研究已经证实黏附作用越好的乳酸菌,其所具有的免疫调节特性越好。如FENG J等[80]从发酵蔬菜中随机选择16株乳酸菌,通过其体外黏附和免疫调节特性挑选出可对抗沙门氏菌(Salmonella)感染的潜在益生菌,结果表明16株菌中鼠李糖乳杆菌P1、植物乳杆菌P2、鼠李糖乳杆菌P3和干酪乳杆菌P2黏附Caco-2细胞的能力最高,且植物乳杆菌P2诱导脾细胞表达TNF-α和IL-12的能力更高,并强烈抑制肠炎链球菌对Caco-2细胞的黏附和侵袭。阴道来源的鼠李糖乳杆菌AD3与阳性对照鼠李糖乳杆菌GG相比对Hep-2和Caco-2细胞表现出更强的体外黏附能力,而且表现出最高水平的抗真菌和细菌的活性[81]。因此乳酸菌的粘附性可作为评价其是否能有效调节免疫性能的重要指标。
乳酸菌在黏附过程中发挥黏附作用的成分主要是脂磷壁酸、黏液结合蛋白、脂多糖和肽聚糖等物质,这些物质被称为黏附素[82]。黏附素与其特异性受体结合,发生一系列细胞变化使乳酸菌定植,如乳酸杆菌和双歧杆菌通过存在于它们表面的脂磷壁酸和动物细胞基质蛋白结构中的受体位点相结合,进而黏附到肠上皮细胞[83];鼠李糖乳杆菌的菌毛蛋白亚基SpaC能够特异性结合位于黏蛋白非还原末端的半乳糖基[84];表层蛋白(Slps)位于细菌细胞壁外的副晶层中,被认为在组织黏附中起重要作用[85]。乳酸菌在进入机体后,通过胃酸的作用大多数乳酸菌会被溶解无法进入小肠,而肠道的蠕动作用又会将黏附性弱的乳酸菌清除肠道,只有黏附性较强的乳酸菌才能在肠道内停留时间较长,进而发挥其更好的作用[86]。因此,根据乳酸菌的黏附特性可高效的挑选出免疫调节特性较好的菌株。
3 总结与展望
乳酸菌可通过激活机体免疫细胞、改变细胞因子含量,利用其黏附性定植于肠道与肠道细胞进行信号交流来达到免疫调节的特性;也可通过调节肠道微生物、调节信号通路及其免疫调节反应来改善Th1/Th2及Th17/Treg细胞的失衡,进而减缓机体的食物过敏症状。
随着越来越多的研究证实,乳酸菌是安全、可食用且可应用于食品加工、医疗、农业等多领域的益生菌。由于其良好的理化特性,研究人员对其展开了更深入的研究,乳酸菌的生物学功能也逐渐成为了研究热点。但大量研究者们对乳酸菌的研究主要集中在乳酸菌菌种的开发和其生物活性方面,而对于乳酸菌与机体免疫系统之间的作用机制仍不太清楚。虽然乳酸菌可以提高机体免疫功能,但也有研究表明某些种类的乳酸菌对机体有致病性,这可能与宿主自身因素(如基因、性别、年龄等)、菌种种类、以及给药剂量、方式等有关。因此,在未来的研究中可注重探讨乳酸菌在治疗食物过敏及免疫调节方面的适用机体及合适的给药方式,也可通过乳酸菌的黏附性探讨其免疫调节的有效性。
[1]GARBACZ K.Anticancer activity of lactic acid bacteria[J].Semin Cancer Biol,2022,86(3):356-366.
[2]SØRENSEN H M, ROCHFORT K D, MAYE S, et al.Exopolysaccharides of lactic acid bacteria: production, purification and health benefits towards functional food[J].Nutrients,2022,14(14):2938.
[3]WELS M,SIEZEN R,VAN HIJUM S,et al.Comparative genome analysis of Lactococcus lactis indicates niche adaptation and resolves genotype/phenotype disparity[J].Front Microbiol,2019,10:4.
[4]HARPER A R, DOBSON R C J, MORRIS V K, et al.Fermentation of plant-based dairy alternatives by lactic acid bacteria[J].Microb Biotechnol,2022,15(5):1404-1421.
[5]TANGYU M, MULLER J, BOLTEN C J, et al.Fermentation of plantbased milk alternatives for improved flavour and nutritional value[J].Appl Microbiol Biotechn,2019,103(23-24):9263-9275.
[6]LI W,GAO L,HUANG W,MA Y,et al.Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans[J].Food Funct,2022,13(6):3690-3703.
[7]SHI Y,CUI X,GU S,et al.Antioxidative and probiotic activities of lactic acid bacteria isolated from traditional artisanal milk cheese from northeast China[J].Probiot Antimicrob Protein,2019,11(4):1086-1099.
[8]MÉNDEZ U T Z,PÉREZ VISÑUK D,PERDIGÓN G,et al.Milk fermented by Lactobacillus casei CRL431 administered as an immune adjuvant in models of breast cancer and metastasis under chemotherapy[J].Appl Microbiol Biotechn,2021,105(1):327-340.
[9]WU J, ZHANG Y, YE L, et al.The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro:A review[J].Carbohydr Polym,2021,253:117308.
[10]CUI X,SHI Y,GU S,et al.Antibacterial and antibiofilm activity of lactic acid bacteria isolated from traditional artisanal milk cheese from northeast China against enteropathogenic bacteria[J].Probiotics Antimicro,2018,10(4):601-610.
[11]LI Z,LI Y,XIAO C,et al.Genomic and metabolic features of the Lactobacillus sakei JD10 revealed potential probiotic traits[J].Microbio Res,2022,256:126954.
[12]LV M, WANG S, YIN H, et al.Probiotic potential and effects on gut microbiota composition and immunity of indigenous gut lactobacilli in Apis cerana[J].Probiotics Antimicro,2022,14(2):252-262.
[13]CHEN L,WANG L,LI J,et al.Antihypertensive potential of fermented milk: the contribution of lactic acid bacteria proteolysis system and the resultant angiotensin-converting enzyme inhibitory peptide[J].Food Funct,2021,12(22):11121-11131.
[14]BASTURK A,ISIK I˙,ATALAY A,et al.Investigation of the efficacy of Lactobacillus rhamnosus GG in infants with cow′s milk protein allergy:a randomised double-blind placebo-controlled trial[J].Probiotics Antimicro,2020,12(1):138-143.
[15]GUADAMURO L,DIAZ M,JIMÉNEZ S,et al.Fecal changes following introduction of milk in infants with outgrowing non-IgE cow′s milk protein allergy are influenced by previous consumption of the probiotic LGG[J].Front Immunol,2019,10:1819.
[16]ZHOU Y, CUI Y, QU X.Exopolysaccharides of lactic acid bacteria:structure,bioactivity and associations:A review[J].Carbohydr Polym,2019,207:317-332.
[17]S,ANLIER N,GÖKCEN B B,SEZGIN A C.Health benefits of fermented foods[J].Crit Rev Food Sci Nutr,2019,59(3):506-527.
[18]SHIN H S,EOM J E,SHIN D U,et al.Preventive effects of a probiotic mixture in an ovalbumin-induced food allergy model[J].J Microbiol Biotechn,2018,28(1):65-76.
[19]MENDONCA C E, ANDREAE D A.Food allergy[J].Primary Care,2023,50(2):205-220.
[20]EIWEGGER T,HUNG L,SAN DIEGO K E,et al.Recent developments and highlights in food allergy[J].Allergy,2019,74(12):2355-2367.
[21]GARGANO D, APPANNA R, SANTONICOLA A, et al.Food allergy and intolerance:a narrative review on nutritional concerns[J].Nutrients,2021,13(5):1638.
[22]TEDNER S G,ASARNOJ A,THULIN H,et al.Food allergy and hypersensitivity reactions in children and adults-A review[J].J Int Med,2022,291(3):283-302.
[23]DE MARTINIS M, SIRUFO M M, SUPPA M, et al.New perspectives in food allergy[J].Int J Mol Sci,2020,21(4):1474.
[24]NODA M, SULTANA N, HAYASHI I, et al.Exopolysaccharide produced by Lactobacillus paracasei IJH-SONE68 prevents and improves the picryl chloride-induced contact dermatitis[J].Molecules,2019,24(16):2970.
[25]HYUNG K E,MOON B S,KIM B,et al.Lactobacillus plantarum isolated from kimchi suppress food allergy by modulating cytokine production and mast cells activation[J].J Funct Food,2017,29:60-68.
[26]ANVARI S,MILLER J,YEH C Y,et al.IgE-mediated food allergy[J].Clin Rev Allergy Immunol,2019,57(2):244-260.
[27]KANAGARATHAM C, EL ANSARI Y S, LEWIS O L, et al.IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy[J].Front Immunol,2020,11:603050.
[28]SAMPSON H A, O′MAHONY L, BURKS A W, et al.Mechanisms of food allergy[J].J Allergy Clin Immun,2018,141(1):11-19.
[29]YU W, FREELAND D M H, NADEAU K C.Food allergy: immune mechanisms,diagnosis and immunotherapy[J].Nat Rev Immunol,2016,16(12):751-765.
[30]HEINE R G.Food allergy prevention and treatment by targeted nutrition[J].Ann Nutr Metabo,2018,72(3):33-45.
[31]CROSS M L,STEVENSON L M,GILL H S.Anti-allergy properties of fermented foods: an important immunoregulatory mechanism of lactic acid bacteria[J].Int Immunopharmacol,2001,1(5):891-901.
[32]PARRISH C P,HAR D,ANDREW BIRD J.Current status of potential therapies for IgE-mediated food allergy[J].Curr Allergy Asthma Rep,2018,18(3):18.
[33]KIM E H,BURKS A W.Food allergy immunotherapy:Oral immunotherapy and epicutaneous immunotherapy[J].Allergy,2020,75(6):1337-1346.
[34]MAJSIAK E,CHOINA M,KNYZIAK-M DRZYCKA I,et al.IgE-Dependent allergy in patients with celiac disease: a systematic review[J].Nutrients,2023,15(4):995.
[35]MONTUORI-ANDRADE A C M,NOLASCO A E,MALACCO N L S O,et al.Lactobacillus delbrueckii UFV-H2b20 increases IFN-γ production and CD39+CD73+Treg cell numbers in lungs,and protects mice against experimental allergic asthma[J].Immunobiology,2022,227(6):152284.
[36]LOKE P, ORSINI F, LOZINSKY A C, et al.Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia(PPOIT-003):a multicentre,randomised,phase 2b trial[J].Lancet Child Adolescent Health,2022,6(3):171-184.
[37]JIANG S,HOU Y,MENG L,et al.Effect of Lactiplantibacillus plantarum HM-22 on immunoregulation and intestinal microbiota in α-lactalbumininduced allergic mice[J].Food Funct,2021,12(19):8887-8898.
[38]GU S M,YANG D,LIU C L,et al.The role of probiotics in prevention and treatment of food allergy[J].Food Science and Human Wellness,2023,12(3):681-690.
[39]SHU S A,YUEN A W T,WOO E,et al.Microbiota and food allergy[J].Clin Rev Allergy Immunol,2019,57(1):83-97.
[40]BERNI CANANI R,PAPARO L,NOCERINO R,et al.Gut microbiome as target for innovative strategies against food allergy[J].Front Immunol,2019,10:191.
[41]IWEALA O I,NAGLER C R.The microbiome and food allergy[J].Ann Rev Immunol,2019,37:377-403.
[42]GAO J,XU K,LIU H N,et al.Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism[J].Front Cell Infect Mi,2018,8:13.
[43]COSTANZO M D,CARUCCI L,CANANI R B,et al.Gut microbiome modulation for preventing and treating pediatric food allergies[J].Int J Mol Sci,2020,21(15):5275.
[44]DUAN C,MA L,YU J,et al.Oral administration of Lactobacillus plantarum JC7 alleviates OVA-induced murine food allergy through immunoregulation and restoring disordered intestinal microbiota[J].Eur J Nutr,2023,62(2):685-698.
[45]CHEN X, WU Y, HU Y, et al. Lactobacillus rhamnosus GG reduces β-conglycinin-allergy-induced apoptotic cells by regulating bacteroides and bile secretion pathway in intestinal contents of BALB/c mice[J].Nutrients,2020,13(1):55.
[46]CUKROWSKA B,CEREGRA A,MACIORKOWSKA E,et al.The effectiveness of probiotic Lactobacillus rhamnosus and Lactobacillus casei strains in children with atopic dermatitis and cCow′s milk protein allergy:A multicenter, randomized, double blind, placebo controlled study[J].Nutrients,2021,13(4):1169.
[47]KELSEN S G,AGACHE I O,SOONG W,et al.Astegolimab(anti-ST2)efficacy and safety in adults with severe asthma: A randomized clinical trial[J].J Allergy Clin Immun,2021,148(3):790-798.
[48]LI N, YU Y, CHEN X, et al.Bifidobacterium breve M-16V alters the gut microbiota to alleviate OVA-induced food allergy through IL-33/ST2 signal pathway[J].J Cell Physiol,2020,235(12):9464-9473.
[49]TIAN X,LIANG X,HE H,et al.Probiotics alleviate food protein allergy in mice by activating TLR4 signaling pathway[J].Mol Nutr Food Res,2023,22:e2200579.
[50]HAJAVI J,ESMAEILI S A,VARASTEH A R,et al.The immunomodulatory role of probiotics in allergy therapy[J].J Cell Physiol,2019,234(3):2386-2398.
[51]RAHBAR SAADAT Y, YARI KHOSROUSHAHI A, POURGHASSEM GARGARI B.A comprehensive review of anticancer,immunomodulatoryand health beneficial effects of the lactic acid bacteria exopolysaccharides[J].Carbohydr Polym,2019,217:79-89.
[52]PLAZA-DIAZ J, RUIZ-OJEDA F J, GIL-CAMPOS M, et al.Mechanisms of action of probiotics[J].Adv Nutr,2019,10(1):S49-S66.
[53]HE F, MORITA H, KUBOTA A, et al.Effect of orally administered non-viable Lactobacillus cells on murine humoral immune responses[J].Microbiol Immunol,2005,49(11):993-997.
[54]NONAKA Y, IZUMO T, IZUMI F, et al.Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production[J].Int Arch Allergy Immunol,2008,145(3):249-257.
[55]PAPARO L,NOCERINO R,D I SCALA C,et al.Targeting food allergy with probiotics[J].Adv Exp Med Biol,2019,1125:57-68.
[56]YIN M,ZHANG Y,LI H.Advances in research on immunoregulation of macrophages by plant polysaccharides[J].Front Immunol,2019,10:145.
[57]KUMAR D,SAHOO S S,CHAUSS D,et al.Non-coding RNAs in immunoregulation and autoimmunity:Technological advances and critical limitations[J].J Autoimmun,2023,134:102982.
[58]ZHANG N, LI C, NIU Z, et al.Colonization and immunoregulation of Lactobacillus plantarum BF_15, a novel probiotic strain from the feces of breast-fed infants[J].Food Funct,2020,11(4):3156-3166.
[59]NIU X X, LI T, ZHANG X, et al. Lactobacillus crispatus modulates vaginal epithelial cell innate response to Candida albicans[J].Chinese Med J,2017,130(3):273-279.
[60]PERDIGÓN G,FULLER R,RAYA R.Lactic acid bacteria and their effect on the immune system[J].Curr Issue Intest Microbiol,2001,2(1):27-42.
[61]ZHANG Z, MAN C, SUN L, et al.Short communication: Complete genome sequence of Lactobacillus plantarum J26, a probiotic strain with immunomodulatory activity[J].J Dairy Sci,2019,102(12):10838-10844.
[62]MIN F,HU J,HUANG T,et al.Effects of Lactobacillus casei NCU011054 on immune response and gut microbiota of cyclophosphamide induced immunosuppression mice[J].Food Chem Toxicol,2023,174:113662.
[63]LEE J, KIM S, KANG C H.Immunostimulatory activity of lactic acid bacteria cell-free supernatants through the activation of NF-κB and MAPK signaling pathways in RAW 264.7 cells[J].Microorganisms,2022,10(11):2247.
[64]YRLID U,SVENSSON M,JOHANSSON C,et al.Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response[J].FEMS Immunol Med Microbiol,2000,27(4):313-320.
[65]MATSUBARA V H,ISHIKAWA K H,ANDO-SUGUIMOTO E S,et al.Probiotic bacteria alter pattern-recognition receptor expression and cytokine profile in a human macrophage model challenged with Candida albicans and lipopolysaccharide[J].Front Microbiol,2017,8:2280.
[66]JIA D J,WANG Q W,HU Y Y,et al.Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206+macrophagesIL-10 activation[J].Gut Microbes,2022,14(1):2145843.
[67]DILNA S V,SURYA H,ASWATHY R G,et al.Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF 4[J].LWT-Food Sci Technol,2015,64(2):1179-1186.
[68]HIDALGO-CANTABRANA C,LÓPEZ P,GUEIMONDE M,et al.Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria[J].Probiot Antimicrob Protein,2012,4(4):227-237.
[69]YU Y,ZONG M,LAO L,et al.Adhesion properties of cell surface proteins in Lactobacillus strains in the GIT environment[J].Food Funct,2022,13(6):3098-3109.
[70]MERCIER-BONIN M,CHAPOT-CHARTIER M P.Surface proteins of Lactococcus lactis: bacterial resources for muco-adhesion in the gastrointestinal tract[J].Front Microbiol,2017,8:2247.
[71]CHUA J, HALE J, SILCOCK P, et al.Bacterial survival and adhesion for formulating new oral probiotic foods[J].Crit Rev Food Sci Nutr,2020,60(17):2926-2937.
[72]LAU L Y, CHYE F Y.Antagonistic effects of Lactobacillus plantarum 0612 on the adhesion of selected foodborne enteropathogens in various colonic environments[J].Food Control,2018,91:237-247.
[73]ROCHA-MENDOZA D, KOSMERL E, MIYAGUSUKU-CRUZADO G, et al.Growth of lactic acid bacteria in milk phospholipids enhances their adhesion to Caco-2 cells[J].J Dairy Sci,2020,103(9):7707-7718.
[74]YAN F,CAO H,COVER T L,et al.Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth[J].Gastroenterology,2007,132(2):562-575.
[75]WANG S,AHMADI S,NAGPAL R,et al.Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut,inflammation and improves physical and cognitive functions:from C.elegans to mice[J].Geroscience,2020,42(1):333-352.
[76]NISHIYAMA K, SUGIYAMA M, MUKAI T.Adhesion properties of lactic acid bacteria on intestinal mucin[J].Microorganisms,2016,4(3):34.
[77]SÁNCHEZ B, DELGADO S, BLANCO-MÍGUEZ A, et al.Probiotics,gut microbiota, and their influence on host health and disease[J].Mol Nutr Food Res,2017,61(1):1600240.
[78]KIM S K,GUEVARRA R B,KIM Y T,et al.Role of probiotics in human gut microbiome-associated diseases[J].J Microbiol Biotechn,2019,29(9):1335-1340.
[79]KAZ′MIERCZAK-SIEDLECKA K,DACA A,FIC M,et al.Therapeutic methods of gut microbiota modification in colorectal cancer management-fecal microbiota transplantation,prebiotics,probiotics,and synbiotics[J].Gut Microbes,2020,11(6):1518-1530.
[80]FENG J, LIU P, YANG X, et al.Screening of immunomodulatory and adhesive Lactobacillus with antagonistic activities against Salmonella from fermented vegetables[J].World J Microbiol Biotechn,2015,31(12):1947-1954.
[81]STIVALA A,CAROTA G,FUOCHI V,et al.Lactobacillus rhamnosus AD3 as a promising alternative for probiotic products[J].Biomolecules,2021,11(1):94.
[82]ALP D, KULEAS,AN H.Adhesion mechanisms of lactic acid bacteria:conventional and novel approaches for testing[J].World J Microbiol Biotechn,2019,35(10):156.
[83]BÖNISCH E,OH Y J,ANZENGRUBER J,et al.Lipoteichoic acid mediates binding of a Lactobacillus S-layer protein[J].Glycobiology,2018,28(3):148-158.
[84]NISHIYAMA K, UENO S, SUGIYAMA M, et al. Lactobacillus rhamnosus GG SpaC pilin subunit binds to the carbohydrate moieties of intestinal glycoconjugates[J].Animal Sci J,2016,87(6):809-815.
[85]JOHNSON-HENRY K C,HAGEN K E,GORDONPOUR M,et al.Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells[J].Cellular Microbiol,2007,9(2):356-367.
[86]LOUIS P,SCOTT K P,DUNCAN S H,et al.Understanding the effects of diet on bacterial metabolism in the large intestine[J].J Appl Microbiol,2007,102(5):1197-1208.